Traditional treatments for malignant tumors mainly include chemotherapy, radiotherapy and hematopoietic stem cell transplantation. However, with the advancements in tumor immunology, immune-targeted therapy, particularly chimeric antigen receptor (CAR) T (CAT-T) cell therapy, has emerged as a promising strategy for cancer treatment. The FDA approved the first CAR-T cell therapyfor B-cell precursor acute lymphoblastic leukemia (ALL) in 2017, making the beginning of CAR-T cell therapy entering an era of fast-paced and innovative research.
A CAR is a fusion protein that incorporates an antibody-derived targeting fragment, which activates a signaling domain in T cells. CAR-T cells can recognize specific surface antigens on tumor cells without requiring antigen processing and presentation. This suggests that CAR-T cell antigen recognition is independent of major histocompatibility complex restrictions. Up to now, CAR-T cell designs have undergone four generations of evolution (Fig. 1). To manufacture CAR-T cells, T cells are collected from the peripheral blood of patients or donors, genetically modified in vitro, and extensively expanded in vitro after expressing CAR. Afterwards, the patient undergoes lymphodepletion chemotherapy to make room for the adoptive CAR-T cells. These genetically engineered CAR-T cells are then injected into the patient's body, where they recognize and bind to target antigen, proliferate rapidly, and exert anti-tumor effects in vivo.
Currently, the FDA has approved six CAR-T cell products, including tisagenlecleucel, axicabtagene ciloleucel, brexucabtagene autoleucel, lisocabtagene maraleucel, idecabtagene vicleucel, and ciltacabtagene autoleucel. These products target the most common antigens, CD19 and B cell maturation antigen (BCMA). In patients with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL), the response rate to CAR-T cell therapy is approximately 80%-90%. In patients with lymphoma, the overall and complete response rates to CAR-T cell therapy range from 50%-80% and 40%-60%, respectively.
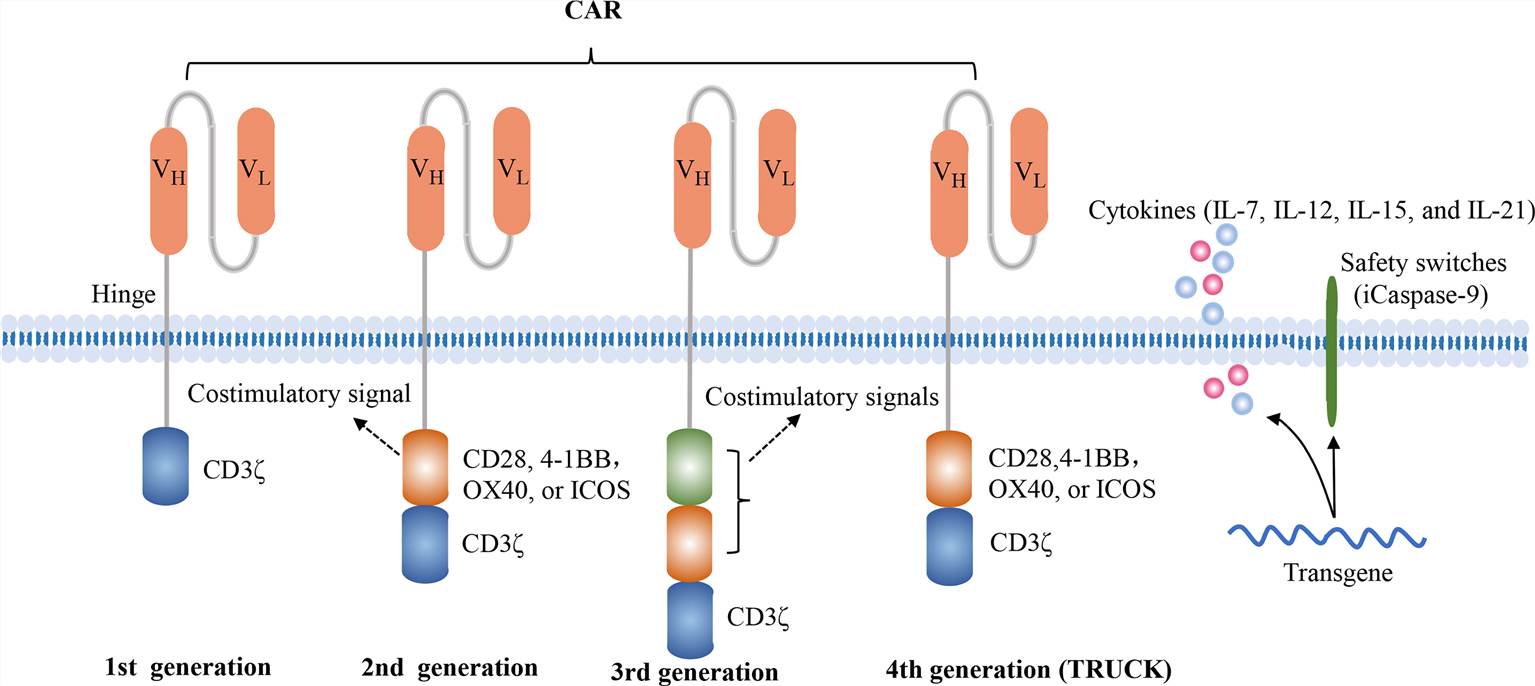 Fig.1 Four generations of CAR constructs (Zhang, 2022)
Fig.1 Four generations of CAR constructs (Zhang, 2022)
CAR-T cell therapy is a significant advancement in the treatment of hematological malignancies and has made remarkable progress in the field. Currently, CD19 and BCMA are the most common targets in CAR-T cell therapy. CAR-T cells targeting CD19 can induce long-term remissions in patients with B-cell malignancies, often with minimal long-term toxicity and potentially cure a subset of patients. Remissions induced by BCMA-targeted CAR-T cells were generally more transient, but often with limited long-term toxicity. Two products have been approved by the FDA for clinical use: (1) Tisagenlecleucel for children with ALL and adult diffuse Sexual large B-cell lymphoma subtype (DLBCL); (2) Axicabtagene ciloleucel for DLBCL. Hematologic malignancies have distinct targets for CAR-T cell therapy (Fig. 2). Unlike B-cell-specific antigens, co-targeting of antigens expressed by other hematopoietic cells may lead to adverse long-term consequences, such as CD33 for the treatment of acute myeloid leukemia, which was withdrawn from the market in 2010 due to severe toxicity (myelosuppression and targeting CD33+ Kupffer cells) after 10 years.
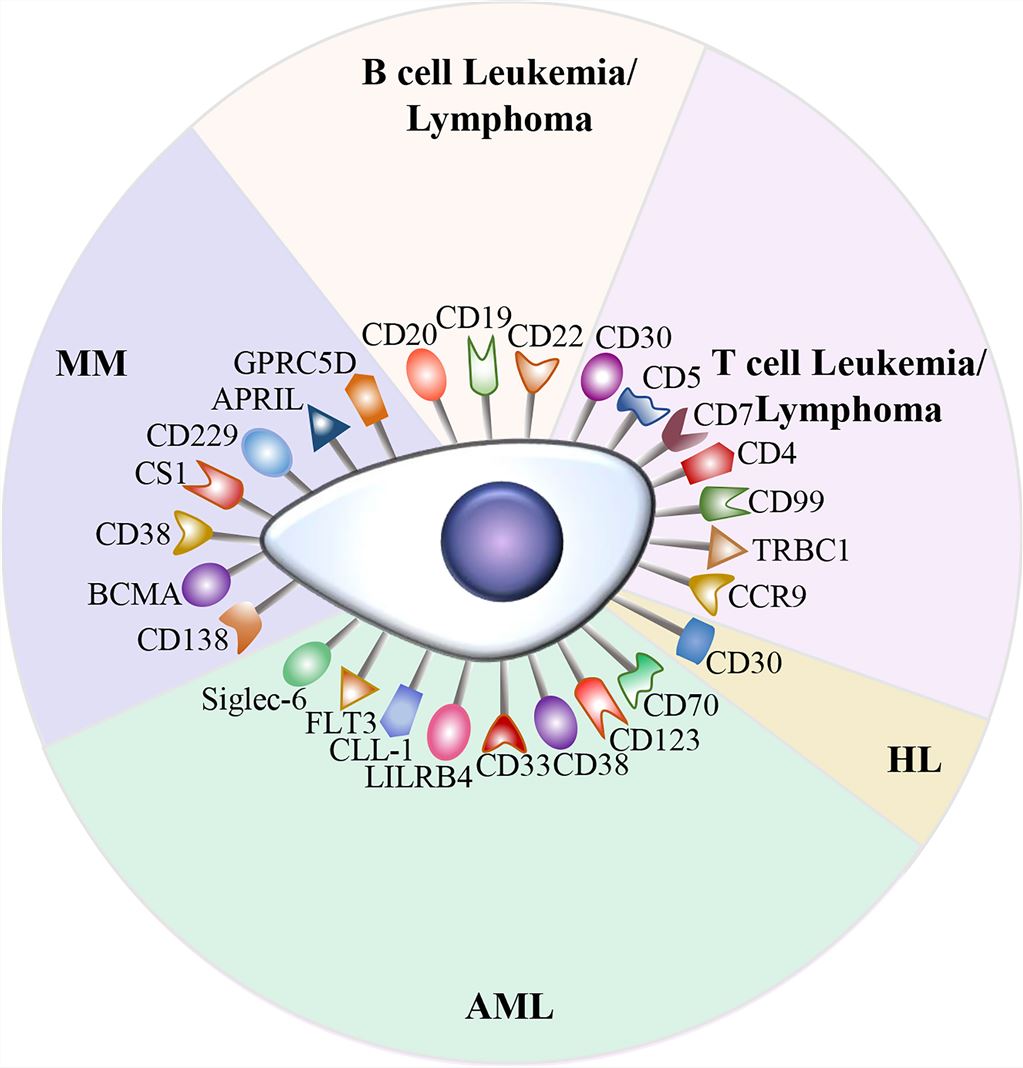 Fig.2 Targets for CAR-T cell therapy (Zhang, 2022)
Fig.2 Targets for CAR-T cell therapy (Zhang, 2022)
In 2007, the FDA approved the first batch of investigational new drug applications for CD19 CAR treatment. CAR-T therapy targeting CD19 has shown significant efficacy in various hematologic malignancies, including ALL, chronic lymphocytic leukemia, and non-Hodgkin's lymphoma. Axicabtagene ciloleucel is a first-in-class anti-CD19 CAR-T cell therapy approved for the treatment of relapsed/refractory large B cell lymphoma, with approximately 60% of patients showing primary treatment resistance within the first year (~15%) or recurrence (~45%). UCART19, one such product studied in children and adults with relapsed/refractory B-cell ALL, demonstrated antileukemic activity and a manageable safety profile. CD19-BBz (86) is a safe and effective anti-CD19 CAR-T cell therapy that produces a robust and durable anti-lymphoma response without neurotoxicity or severe cytokine release syndrome (CRS).
BCMA, a member of the tumor necrosis factor protein superfamily, is expressed primarily by malignant and normal plasma cells, as well as some mature B cells, making it a potential target in multiple myeloma (MM). CAR T- cell therapy targeting BCMA has shown activity in MM. G protein-coupled receptors, class C, group 5, member D (GPRC5D) have been identified as immunotherapeutic targets in MM. In the GPRC5D-targeted CAR T cell therapy study (MCARH109), it has been demonstrated that GPRC5D is a key target for active immunotherapy in MM. In the CARTITUDE-1 trial (NCT03548207), BCMA-targeted CAR-T cell therapy developed neurocognitive and hypomotor dyskinesias characteristic of Parkinson's disease. ALLO-715 is a first-in-class allogeneic anti-BCMA CAR-T cell therapy designed to eliminate graft-versus-host disease and minimize CAR-T rejection. Preliminary results from its trial show that allogeneic CAR-T cell therapy is feasible and safe for MM.
The efficacy of CAR-T cell therapy for solid tumors is limited by several factors, including the number, persistence, and function of transferred T cells, as well as the heterogeneous and immunosuppressive nature of the tumor microenvironment (TME). Increasing doses of CAR-T cells has resulted in significant antitumor activity, but it also been associated with patient mortality, the cause of which is unknown but could be related to macrophage activation syndrome or immune effector cell-associated neurotoxicity. Adoptively transferred CAR-T cells targeting solid tumors undergo limited homeostatic expansion in the blood and encounter tumor antigens in the immunosuppressive TME, where CAR-T cells may not be present in sufficient numbers to eradicate the disease. Nanoparticle RNA and peptide vaccines offer another approach to enhance the in vivo expansion of CAR-T cells targeting solid tumors. Trials evaluating CAR-T cell therapy in solid tumors are also underway. Many factors hinder the manufacture of effective CAR-T cells for solid tumors, including but not limited to the lack of tumor-specific antigens and immunosuppressive TME. There are fewer than a dozen viable CAR-T cell targets, and finding targets with high homologous expression in tumors but not in healthy tissues (to limit on-target, off-tumor toxicity) is difficult.
While CAR-T cell therapy has achieved remarkable efficacy, it is also accompanied by some serious toxic side effects, which may compromise its efficacy and even endanger life. Side effects of CAR-T cells can involve almost any major organ system. Patients can experience respiratory, cardiovascular, hematologic, renal, neurologic, and gastrointestinal toxicities ranging from mild to life-threatening. The therapeutic steps for CAR-T cytotoxicity management are shown in Fig. 3.
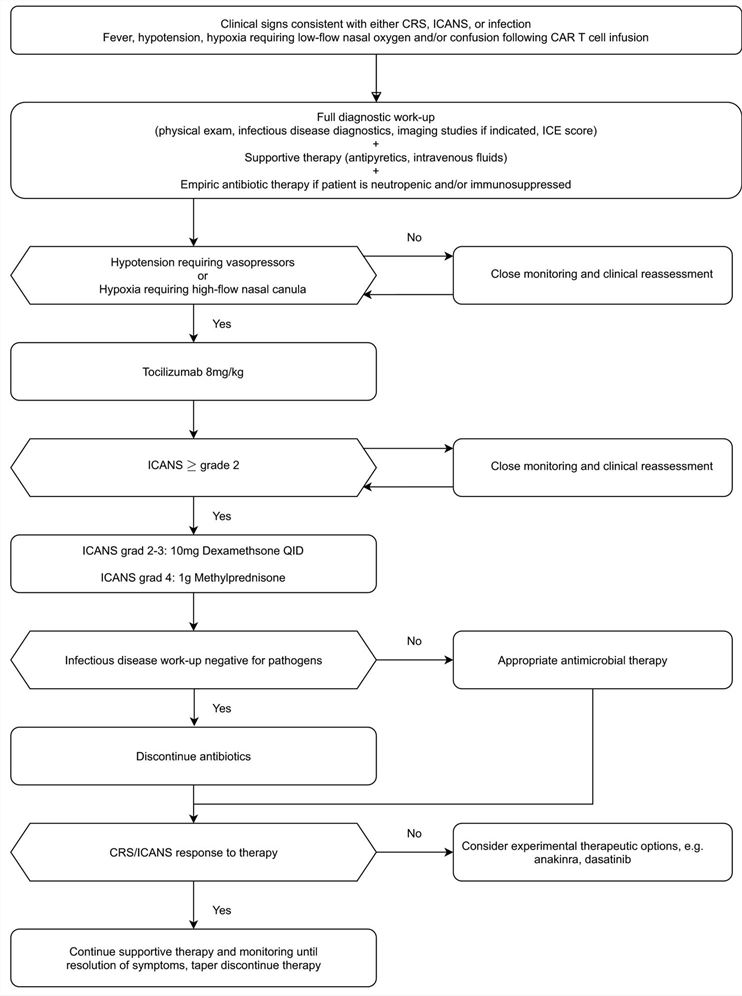 Fig.3 Therapeutic steps for CAR-T cytotoxicity management (Shimabukuro-Vornhagen, 2022)
Fig.3 Therapeutic steps for CAR-T cytotoxicity management (Shimabukuro-Vornhagen, 2022)
The most common complication is a systemic inflammatory response caused by excessive activation of CAR-T cells and endogenous immune cells such as macrophages and dendritic cells. It is characterized by high fever, sinus tachycardia, hypotension, hypoxia, decreased cardiac function, and other organ dysfunction (Fig. 4).
Neurotoxicity is a heterogeneous and poorly understood disease with varying clinical presentation and severity. Patients may experience confusion, hallucinations, cognitive deficits, tremors, ataxia, speech disturbances, nerve palsies, focal motor or sensory deficits, myoclonus, lethargy, seizures, etc. Deaths due to CRS and neurotoxicity have been reported (Fig. 4).
It is reversible in most cases, such as various arrhythmias, electrolyte disturbances, abnormal elevation of liver enzymes and bile enzymes, tumor lysis syndrome, etc.
Common adverse events were anemia, thrombocytopenia, neutropenia, and disseminated intravascular coagulation. Hypogammaglobulinemia and B-cell hypoplasia are common after anti-CD19 CAR-T cell infusion.
Patients may become susceptible to viral, bacterial, and invasive fungal infections.
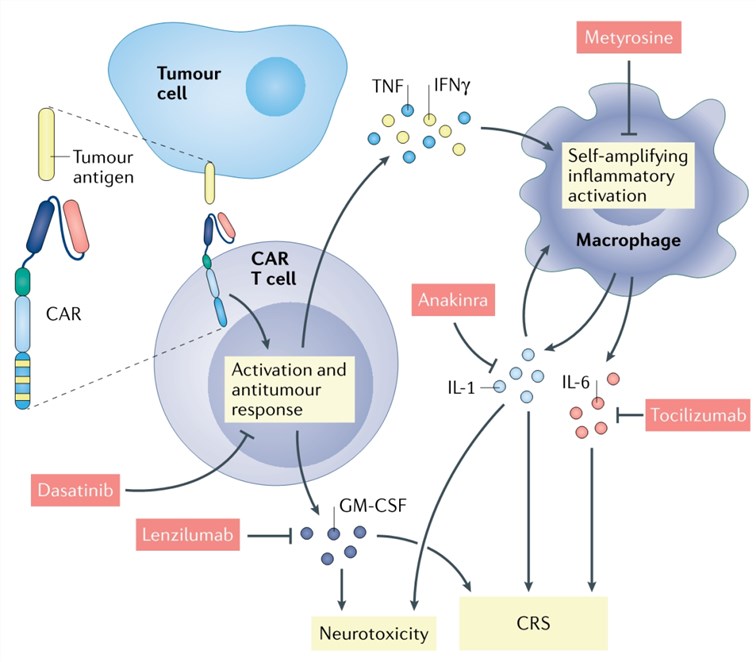 Fig. 4. Mechanism and therapy of CAR-T cell-induced CRS and neurotoxicity (Larson, 2021)
Fig. 4. Mechanism and therapy of CAR-T cell-induced CRS and neurotoxicity (Larson, 2021)
CAR-T cells that have enhanced proliferation and survival are beneficial in a variety of diseases. Limiting exhaustion while enhancing the proliferative capacity of CAR-T cells also benefits antitumor responses. However, there are still some challenges to overcome in future research, including overcoming antigen loss, exhaustion, and immunosuppressive TME. Strategies to prevent extra-tumor reactions in CAR-T cell therapy include: (1) Preclinical evaluation of the safety of CAR therapy which involves analyzing of information on the expression of target antigens on the surface of important cells, in vivo performance of source monoclonal antibodies, and so on; (2) Reactivity measures such as inhibitory CAR, combined antigen recognition, mutating CAR recognition domain, limiting the persistence and function of CAR-T cells in vivo, and alternative routes of T cell administration; (3) Reactive measures like inducible suicide genes and antibodies for using in selective CAR-T cell depletion.
In recent years, a series of manufacturing innovations, such as gene transfer, improving the effector cell type, and allogeneic CARs, have been carried out to address the clinical toxicity associated with CAR-T cell therapy. CAR-T cell research has rapidly entered the clinic, but there are still many obstacles to overcome. Finding new mechanisms and new targets to improve efficacy, reduce toxicity, and reduce drug resistance remains a challenge for future research. The innovation of CAR-T cell engineering is the focus of future research with the aim of improving the clinical efficiency of hematological malignancies and solid tumors, overcoming current limitations such as antigen escape, CAR-T cell trafficking, tumor infiltration, immunosuppressive microenvironment, and CAR-T cell-related toxicity, etc.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION