Adoptive cell transfer (ACT) is a form of transfusion therapy involving the infusion of large numbers of somatic cells especially immune cells with the aim of eliminating, or at least controlling malignancies or infectious diseases. Indeed, ACT can lead to tumor regression and even eradication in some patients with advanced cancers. ACT requires immune cells collected from patients or donors or differentiated from stem cells. These immune cells are then activated and expanded in vitro, subjected to gene modification, and then infused into the patients through a peripheral vein or regional artery. A variety of immune cells, including T cells, natural killer (NK) cells, and dendritic cells (DCs), can be used in ACT although their functional mechanisms are different. In recent years, due to the advancement of cellular and molecular biology techniques, such as T cell extraction, expansion and activation ex vivo and genetic engineering techniques, ACT of genetically engineered T cells is becoming a viable therapeutic option for cancer patients who are refractory to conventional therapy.
T Cells and The Role of T Cell Receptors
T cells (also known as T lymphocytes) are widely distributed in tissues and tumor environments. They play a central role in cell-mediated immunity and can mediate longevity, antigen specificity, effectors and immune memory responses. The difference between T cells and other lymphocytes is that there are T cell receptors (TCRs) on the surface of T cells. TCR is a multi-subunit transmembrane complex that mediates antigen-specific activation of T cells. TCR consists of two different polypeptide chains, α chain and β chain. Each chain has an N-terminal variable region and a constant region. These chains are linked by disulfide bonds, and each receptor provides a single antigen binding site.
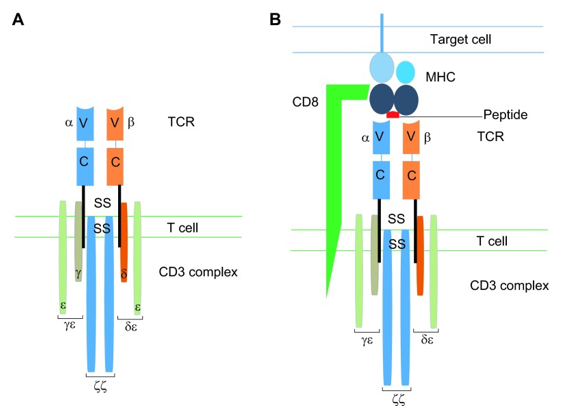 Fig. 1. Structure and function of the TCR.1
Fig. 1. Structure and function of the TCR.1
TCR endows T cell with antigen specificity by recognizing antigen ligands, which are composed of short adjacent amino acid sequences of proteins presented on target cells by major histocompatibility complex molecules. The auxiliary adhesion molecules expressed by T cells, such as CD4 of MHC II and CD8, of MHC I, are also involved. TCR interacts with the ligand by contacting with MHC molecules and antigen peptides. Signal transduction is mediated by a related invariant CD3 complex, which is composed of four different CD3 proteins forming two heterodimers (CD3δε and CD3γε) and one homodimer (CD3ζζ).
After contacting with homologous peptides presented by MHC class I molecules, simple CD8+ cytotoxic T cells proliferated rapidly and obtained phenotypic and functional characteristics, allowing them to act as effector T cells. These cells eliminate antigen expression by apoptosis-inducing ligands or the release of cleavage particles. In addition, long-term memory T cells capable of self-renewal are produced, allowing rapid expansion in the presence of the target antigen and providing a sustained and lasting response to re-exposure. The function of T cells as the coordinator and effector of adaptive immune response is guided by the specificity of TCR.
Genetically Modified T Cells in Cancer Immunotherapy
The concept of transferring T cells to patients (adoptive T cell transfers) to treat diseases has been established over the years through ex vivo manipulation, expansion, and reinfusion of T cells targeting specific viruses, for example for the treatment of viral infections, such as cytomegalovirus or Epstein Barr virus infection after hematopoietic stem cell transplantation. As mentioned above, rare tumor antigen-specific T cell populations do exist and can be specifically separated from tumor sites, known as tumor infiltrating lymphocytes (TILs). TIL can be isolated from resected tumor tissue and cultured, activated and amplified in vitro. It shows promising clinical efficacy, especially in the treatment of melanoma, and supports the therapeutic potential of tumor-specific T cells.
Another option for these methods now is to produce convincing clinical data, provided that the antigen specificity of T cells can be manipulated by genetic modification and redirected to successfully target tumor-expressed antigens. In particular, T cells can be designed to express modified TCR (so-called TCR therapy) or protein fusion derived chimeric antigen receptor (CARS), with enhanced antigen specificity. These methods can overcome the basic limitations associated with central and peripheral tolerance and produce more effective T cells targeting tumors without the need for ab initio T cell activation in patients.
Genetically modified TCR therapy is based on altering the specificity of T cells by expressing specific TCR α and β chains, which mediate antigen recognition. The tumor-specific TCR α and β chains were recognized, isolated and cloned into the transduction vector, and the transduction of T cells produced tumor antigen-specific T cells.
In order to produce a successful tumor-specific TCR, it is necessary to identify the appropriate target sequence. This may be isolated from rare tumor reactive T cells or, where it is impossible, alternative techniques can be used to produce highly active anti-tumor T cell antigens. One method is to immunize transgenic mice expressing human leukocyte antigen system with human tumor protein to produce T cells expressing TCR against human antigen. Another method is allogeneic TCR gene transfer, in which tumor-specific T cells are isolated from patients undergoing tumor remission, and reactive TCR sequences are transferred to T cells from another patient, who is the same as the former patient but does not respond. Finally, the in vitro technique can be used to change the sequence of TCR and enhance its antitumor activity by increasing the interaction intensity (affinity) between weakly reactive tumor specific TCR and target antigen.
Combining antibody-like recognition with T cell activation, CARs consist of antigen binding domains (usually from antibodies), transmembrane domains (used to anchor CAR to T cells), and one or more intracellular signal domains. These signal domains induce persistence, transport and effect functions in transduced T cells. The sequences used to define the antigen targeting motifs of CAR usually come from monoclonal antibodies, but ligands and other receptors can also be used. Viral- and non-viral-based genetic engineering tools have been used to generate CAR-expressing T cells (CAR-T cells), resulting in permanent or transient expression of therapeutic genes (Table 1). However, most of the current clinical trials developing modified T cells employ the latest generations of retroviral (gamma retroviral and lentiviral) vectors.
Table 1 Strategies used to genetically modify T cells ex vivo to express CARs
| Transgene Insertion | Transgene Delivery | Types | Features and Observations | |
| retroviral vectors | non-targeted integration | ex vivo transduction | gamma retrovirus | safe, optimized, and FDA-approved protocols; the production of the therapeutic cells is expressive |
| lentivirus | transgene size limitation | |||
| transposase enzymes |
non-targeted integration |
ex vivo electroporation | sleeping beauty, piggyBac | FDA-approved protocols, more economical than viral vectors, but less developed technology; transposase have to be electroporated along with the donor DNA (plasmid or minicircles) |
| mRNA | non- integrative | ex vivo electroporation | N/A | fast and economical method to produce CAR-T cells; the transgene expression is rapidly diluted over the expansion of the T cells; ideal when first introducing a novel CAR into patients |
| non-integrative lentivirus | non-integrative, episomal | ex vivo transduction | NILV-S/MAR | the transient expression can be extended up to 30 days; the production of the nonintegrative lentivirus is expensive and will still require constant re-dosing |
| endonuclease enzymes | targeted integration | ex vivo electroporation | gene editing | directed transgene insertion into the host cell genome; ability to ablate specific host cell genes; endonucleases have to be electroporated along with donor DNA (AAV or linear dsDNA); further protocol optimization is required |
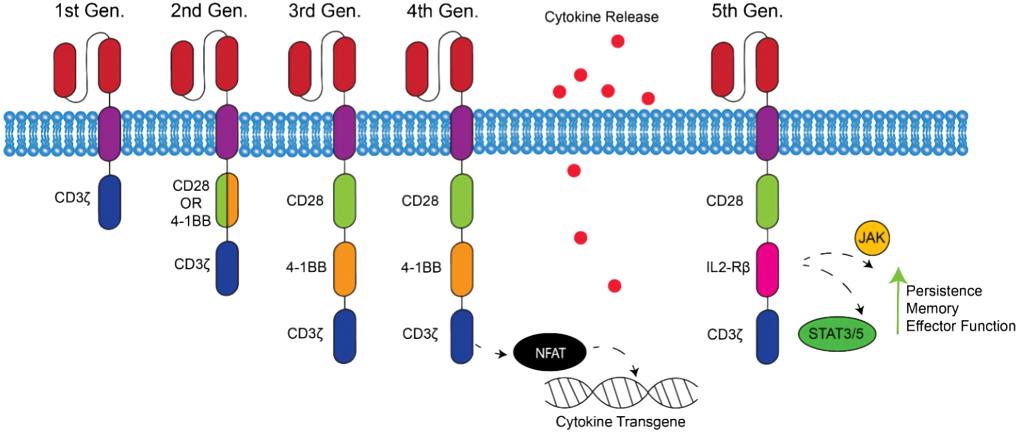 Fig. 2 Five CAR generations.2
Fig. 2 Five CAR generations.2
Single structure from the CD3 ζ- chain or FcεRIγ from the intracellular domain for the first-generation of CARs is the typical characteristic, which is the primary transmitter of signals from endogenous T cell receptor (TCR). However, these CAR-T cells could not produce enough interleukin-2 (IL-2), so in order to kill tumor cells, it was necessary to administer exogenous IL-2. Therefore, the first generation of CAR-T cell therapy transfected with single-chain receptors benefitted substantially from the accompanying administration of cytokines. CAR-T cells recognize many types of antigens, not only proteins, but also carbohydrates and glycolipids typically expressed on the surface of tumor cells. Unlike TCR recognition, antigens do not need to be processed and presented by MHC, so the same CAR-based method can be used in all patients who express the same tumor antigen, regardless of the HLA type.
Dual signal is the typical characteristic for T cell activation. Three different receptor types --T-cell antigen receptors, cytokine receptors and co-stimulatory receptors are included in this progression. The first signal is the special signal that triggered by the TCR, recognizing the antigenic peptide-MHC complex on the surface of antigen-presenting cells. The second signal is the co-stimulatory signal, produced by a co-stimulatory molecule such as CD28/B7, which promotes the IL-2 synthesis to complete the activation of T cells and avoid apoptosis. Naïve T cells cannot perform their normal role if the co-stimulatory signal is absent, and it doesn't work even if the T cells are stimulated by the antigen. Therefore, CARs that only include the CD3ζ sequence cannot activate CAR-T cells without a co-stimulatory signal. Accordingly, the second-generation CARs added intracellular signaling domains from various co-stimulatory protein receptors to the cytoplasmic tail of the CARs to provide additional signals to the T cell, such as CD28 or CD137 (4-1BB and CD134(OX40)), which can improve the proliferation, cytotoxicity and sustained response, and prolong the life of CAR-T cells in vivo. CD28-mediated co-stimulation is very important in the regulation of proliferation and survival for lymphocytes, and plays a key role for the establishment of memory cells and effector cells. CD134 can sustain proliferation and strengthen IL-2 production. CD137 can maintain the response signal of T cells, which is vital in the survival of T cells and the memory of CD8+ T cells.
The third-generation CARs were made by combining multiple signaling domains, such as CD3ζ-CD28-OX40 or CD3ζ-CD28-41BB, to augment potency with stronger cytokine production and killing ability. For example, α-CD19-CD3ζ-41BB cells in chronic lymphoblastic leukemia show complete remission to infiltrating lytic cancer tissues. Better yet, a small number of cells can be used as memory phenotypes to prevent tumor recurrence. Despite the significant therapeutic effect, the emerging uncontrollable activity associated with more antineoplastic effects leads to life-threatening dissolution as the most critical side effect or toxicity. Including clinically significant release of proinflammatory cytokines, pulmonary toxicity, multiple organ failure and eventual death.
The fourth-generation CARs were generated by adding IL-12 to the base of the second-generation constructs, and are known as T cell redirected for universal cytokine-mediated killing (TRUCKs). TRUCKs augment T-cell activation, activate and attract innate immune cells to eliminate antigen-negative cancer cells in the targeted lesion. It would be worthwhile to explore the role of TRUCKs in shaping the tumor environment by the inducible release of transgenic immune modifiers. Such TRUCK T cells can also treat viral infections, metabolic disorders and auto-immune diseases.
New strategies have been developed to design novel CAR constructs. Dual CARs co-express two different CARs in one cell. Tandem CARs (TanCAR) contain two different scFvs in a single CAR molecule that can either be stacked in series or as a looped structure. Dual and tandem CARs function as OR-gate circuits: CAR-T cells are activated following recognition of antigen A or antigen B. Combinatorial CARs combine two constructs: one bears the CD3ζ signaling motif and the other bears the costimulatory signaling domain. Synthetic Notch (syn-Notch) receptors induce the transcription of a CAR after antigen recognition of their cognate antigen. Combinatorial and syn-Notch CARs function as AND-gate circuits: CAR-T cells are fully activated only when both antigen A and antigen B are recognized. On-switch CAR-T cells are inactive until specific activating agents are added, assembling a fully functional receptor. Inhibitory CARs (iCAR) inhibit T cell activation following antigen recognition in normal cells. Universal or switchable CAR-T cells remain inactive until antibody-based molecules targeting a tumor antigen are supplied to reconstitute a fully active CAR construct.
Over 400 clinical trials of CAR-T cell therapy are undergoing, but only two autologous CAR-T cell therapies were approved by the Food and Drug Administration (FDA) and the Europe Union. One is for the treatment of pediatric patients and young adults with refractory or relapse (R/R) B cell precursor acute lymphoblastic leukemia, and the other for the treatment of adult patients with R/R large B cell lymphoma. In common, both are CD19-specific CAR-T cell therapies lysing CD19-positive targets.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION