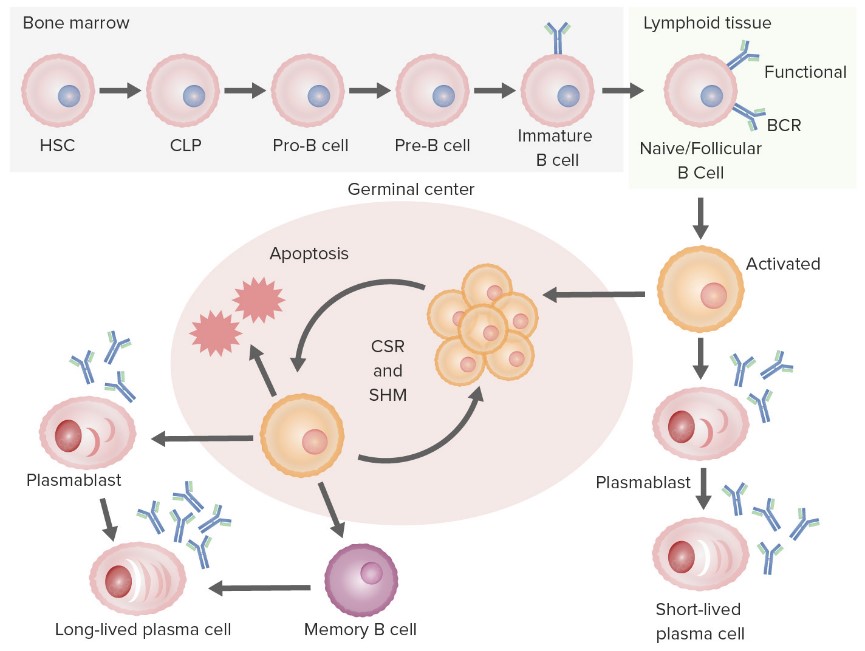
B cells are a type of lymphocyte primarily responsible for the humoral immune response, recognizing and clearing foreign antigens by secreting antibodies. The source of B cells is multipotent hematopoietic stem cells (HSCs), which differentiate into common lymphoid progenitor cells (CLPs) in the bone marrow and then further differentiate into pro-B cells, pre-B cells, immature B cells, and mature B cells. In this process, the immunoglobulin (Ig) gene of B cells undergoes VDJ recombination to generate diverse B cell receptors (BCRs), which are screened and edited by self-antigens to avoid autoimmune reactions.
Mature B cells leave the bone marrow and enter peripheral lymphoid organs, such as the spleen and lymph nodes, where they encounter specific antigens and are helped by helper T follicular helper cells (Tfh) to enter the germinal center. In germinal centers, B cells differentiate into centroblasts and centrocytes and migrate between dark and light zones. Here, somatic hypermutation (SHM) and class switch recombination (CSR) occur in the Ig gene of B cells to improve the affinity and function of the antibody. Finally, selected and optimized B cells differentiate into plasma cells or memory B cells, which are responsible for the production of large amounts of antibodies or long-term memory immunity, respectively.
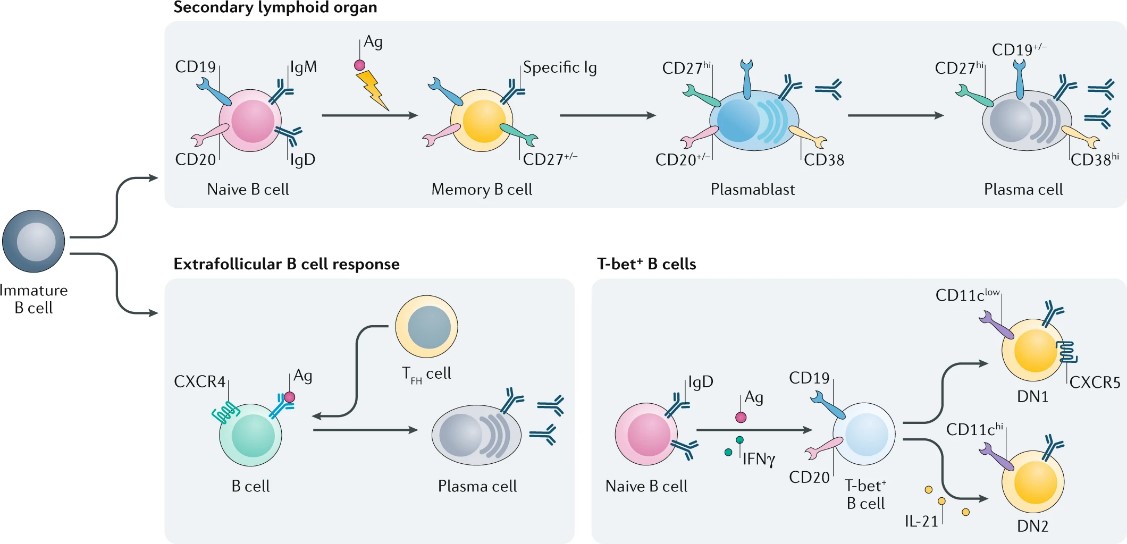 Fig.1 B cell biology
Fig.1 B cell biology
A recent study found that, in addition to developing in peripheral lymphoid organs, B cells can also develop in the meninges and undergo negative selection mediated by Central nervous system (CNS)-specific antigens. This discovery reveals the important role of B cells in neuroimmune regulation and provides new ideas for the diagnosis and treatment of related diseases.
According to different criteria, B cells can be classified in different ways. According to developmental stage, B cells can be divided into stem cells, progenitor cells, precursor cells, immature cells, transition cells, mature cells, activated cells, plasmablasts, plasma cells, and memory cells. These stages reflect the development of B cells from the bone marrow to the peripheral lymphoid organs and their differentiation after encountering antigens. According to surface markers, B cells can be divided into different subgroups, such as B1 cells, marginal zone B cells, follicular B cells, and regulatory B cells, which have different functions and distributions. For example, B1 cells mainly exist in the abdominal cavity and thorax and participate in innate immunity and anti-polysaccharide antigen responses. Marginal zone B cells mainly exist in the marginal zone of the spleen, and participate in T-cell-independent antigen responses. Follicular B cells mainly exist in lymph nodes and splenic follicles and participate in T-cell-dependent antigen responses. Regulatory B cells mainly exist in peripheral blood and lymph nodes and participate in immune tolerance and suppression of inflammatory responses.
According to the surface immunoglobulin (Ig) type, B cells can be divided into different classes, such as IgM+ B cells, IgD+ B cells, IgG+ B cells, IgA+ B cells, and IgE+ B cells. These groups show how class switch recombination (CSR) happens in B cells during development or after they come into contact with antigens. They also show how different types of Ig help the immune system respond. For example, IgM is the earliest antibody produced, with low affinity and high valency. IgD is one of the main surface receptors of mature B cells and can regulate the activation and differentiation of B cells. IgG is the most common serum antibody, with a high affinity and multiple functions. IgA is the most important antibody in mucosal immunity and can neutralize microorganisms and toxins. IgE is an antibody involved in parasitic infection and allergic reactions and has the effect of activating mast cells and basophils.
B cell-mediated immune response refers to the process by which B cells exert humoral immune function by recognizing and binding antigens and secreting antibodies. B cell-mediated immune responses can be divided into two types.
One is a B-cell immune response to a T-cell-dependent antigen (TD antigen), which is usually a protein or polypeptide and requires the cooperation of B cells and T cells to generate an effective immune response. B cells first specifically bind antigens through the B cell receptor (BCR), generating the first signal. Then B cells internalize, process, and present the antigens to T cells, which are specific to T cell receptors (TCRs) and MHC class II molecules. The binding of CD40L on the T cell surface to CD40 on the B cell surface generates a second signal; Finally, under the action of cytokines secreted by T cells (such as IL-4, IL-5, IL-6, etc.), the B cells proliferate, and differentiate into plasma cells or memory B cells.on the T cell surface to CD40 on the B cell surface generates a second signal. Finally, under the action of cytokines secreted by T cells (such as IL-4, IL-5, and IL-6), the B cells proliferate and differentiate into plasma cells or memory B cells.
The other is a B-cell immune response to a T-cell-independent antigen (TI antigen). TI antigens are usually small molecular compounds of polysaccharides, lipids, or repetitive epitopes that can directly activate B cells without the assistance of T cells. TI antigens can be divided into TI-1 and TI-2 antigens. TI-1 antigen has strong mitogen activity, can activate most mature and immature B cells, induce polyclonal B cell proliferation and differentiation, and mainly produces IgM2, such as lipopolysaccharide (LPS). The TI-2 antigen has a highly repetitive epitope structure that can cross-link a large number of BCRs, generate a strong first signal, activate specific mature B cells, and mainly produce IgM and IgG2, such as pneumococcal polysaccharides.
B cell-based immunotherapy is a cancer immunotherapy approach that uses B cells as tools or targets. B cells are lymphocytes that can secrete antibodies, present antigens, regulate immune responses, and form immune memory. B cells have a dual role in cancer, both inhibiting tumor growth and metastasis and promoting tumor escape and drug resistance. Therefore, B cells are important subjects of study for drug and therapy development.
Monoclonal Antibodies Targeting B cells: This method uses monoclonal antibodies (mAb) to specifically recognize and bind to molecules on the surface of B cells, thereby activating or inhibiting B cell functions, enhancing or reducing B cell-mediated immune responses, and killing or cleaning abnormal or malignant B cells. For example, using mAbs targeting B-cell maturation antigen (BCMA), a molecule specifically expressed by multiple myeloma (MM) cells, can effectively eliminate MM cells..
Adoptive Transfer of B cells: This method uses exogenous or endogenous B cells to perform operations such as expansion, activation, and genetic modification in vitro, and then infuse them back into the patient, thereby enhancing the body's immune response to the tumor. For example, chimeric antigen receptor-modified B cells (CAR-B cells) can recognize and kill tumor cells expressing specific tumor antigens.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION