The macrophage is an important immune cell with multiple functions such as phagocytosis, antigen presentation, and cytokine secretion. There are many sources of macrophages, which can be divided into two categories: one is circulating monocytes differentiated from hematopoietic stem cells in the bone marrow, and they migrate to tissues after circulating in the blood to become tissue-specific macrophages. One type is primitive macrophages generated during embryonic development in the embryonic region near the yolk sac, fetal liver, or dorsal aorta, which exist independently of monocytes in adulthood. Macrophages have distinct phenotypes and functions in different tissues and organs and can be polarized into pro-inflammatory M1 or anti-inflammatory M2 phenotypes depending on stimuli and environmental factors. Macrophages act as a bridge in the immune system, participating in the defense response of innate immunity and regulating the activation and memory of adaptive immunity. In recent years, macrophage-based immunotherapy has become a promising and potential cancer treatment. Because macrophage-based immunotherapy can use the phagocytic function of macrophages to directly destroy tumor cells, thereby inhibiting tumor growth and metastasis. In addition, the antigen presentation function of macrophages can be used to activate other immune cells, such as T cells, thereby inducing a specific immune response and enhancing the body's ability to monitor and eliminate tumors.
Macrophages have diverse distributions throughout the body. Depending on their residence tissue, these heterogeneous tissue resident macrophages are divided into various subpopulations with specific names and distinct functions, including Kupffer cells in the liver, red pulp macrophages in the spleen, osteoclasts in the bone, alveolar macrophages in the lung, and microglia in the brain. In another dimension, macrophages are classified into two major subsets depending on their different functional programs in response to environmental stimuli. Classically activated M1 macrophages and alternatively activated M2 macrophages are derived from the primary macrophages when stimulated with pathogen and anti-inflammatory signals, respectively.
Macrophage differentiation is a complex process influenced by multiple factors, including signaling molecules, growth factors, transcription factors, epigenetic and post-transcriptional mechanisms and changes, and niche signals such as cytokines, cell-cell contact substances, and metabolites. Macrophages can be classified according to their function and activation and are divided into two subtypes: classically activated M1 macrophages and alternatively activated M2 macrophages. M1 macrophages are mainly activated by pathogens, lipopolysaccharide (LPS), granulocyte macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor alpha (TNF-α), and type 1 helper T (Th1) cytokine interferon gamma (IFN-γ). They have strong antibacterial and antitumor abilities and can release a large number of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, as well as reactive oxygen species (ROS) and nitric oxide (NO). They are also capable of efficient antigen presentation and express high levels of markers such as major histocompatibility complex class II (MHC II), CD68, CD80, and CD86. M2 macrophages are mainly activated by interleukin 4 (IL-4), IL-10, IL-13, macrophage colony-stimulating factor (M-CSF), and glucocorticoids. They have the functions of anti-inflammation, promoting tissue repair and remodeling, regulating adaptive immunity, etc., and can release a large number of anti-inflammatory cytokines, such as IL-10, TGF-β, etc. They are also capable of expressing high levels of markers such as CD163, CD206, and CD209.
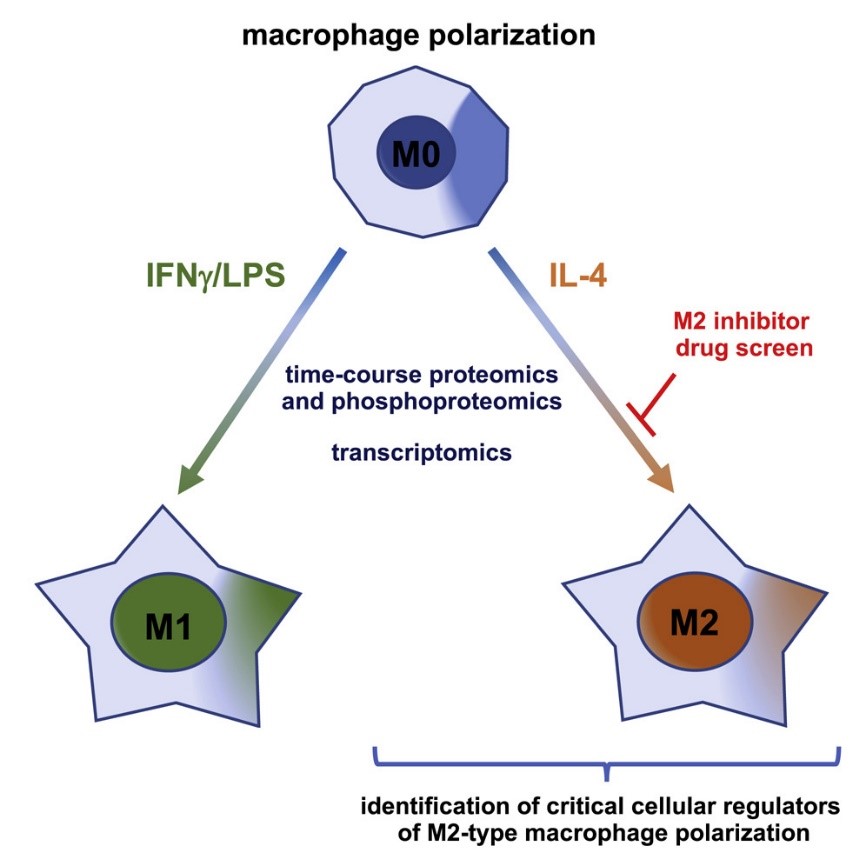 Fig.1 Macrophage polarization (He, 2021)
Fig.1 Macrophage polarization (He, 2021)
Macrophage-mediated immune response refers to the process by which macrophages participate in humoral and cellular immunity by engulfing and presenting antigens and activating T cells and B cells. Macrophages can recognize and bind different ligands, such as pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), Fc receptors, and complement receptors, thereby modulating their phenotype and function. Macrophages can also secrete a variety of cytokines, such as TNF-α, IL-1, IL-6, IL-10, IL-12, and IL-23, which affect the inflammatory response and immune regulation.
The immune response that macrophages mediate is crucial for fighting infection, fighting tumors, and repairing tissue, but it can also result in immune-related diseases like autoimmune diseases, chronic inflammatory diseases, and tumor immune escape. Therefore, modulating macrophage polarization and activation states is a potential therapeutic strategy. For example, the CSF-1/CSF-1R pathway is an important signaling pathway that regulates macrophage survival, proliferation, differentiation, and function. CSF-1 is a stimulatory factor that acts primarily on CSF-1R, a tyrosine kinase receptor mainly expressed in the mononuclear phagocyte system. The CSF-1/CSF-1R pathway plays an important role in normal physiological and pathological processes, especially in the tumor microenvironment, where it can promote the polarization of tumor-associated macrophages (TAMs) into an M2-type phenotype, thereby inhibiting the infiltration and activation of T cells that promote tumor growth, invasion, and metastasis. Therefore, targeting the CSF-1/CSF-1R pathway is a potential anti-tumor immunotherapy approach.
As mentioned above, macrophages are a type of white blood cell that plays an important role in the body's immune system by engulfing and digesting microorganisms, removing debris and dead cells, and stimulating other cells involved in immune function. Macrophages can be found in almost all human tissues, some are fixed in certain tissues, such as lymph nodes and intestines, and some can travel in loose connective tissue spaces. Macrophages play a key role in enabling lymphocytes to recognize foreign particles by engulfing and processing them, thereby determining the specificity of the immune response. Because of their multiple functions in the immune system, macrophages are important subjects of study for drug and therapy development.
Monoclonal antibodies are antibodies that can specifically recognize and bind to target molecules, and they can be used to treat certain diseases, such as cancer, autoimmune diseases, and infectious diseases. Monoclonal antibody therapy against macrophages is a strategy that uses monoclonal antibodies to regulate or eliminate the function of macrophages, and they can be used to treat diseases such as inflammation, fibrosis, and tumors related to macrophages. For example, a monoclonal antibody called CD47 can block the macrophages' "don't eat me" signal to tumor cells, thereby enhancing the macrophages phagocytosis of tumor cells.
Adoptive cell transfer is a method of transplanting engineered or activated immune cells into a recipient, which can be used to enhance or restore the recipient's immune function. The transplanted immune cells can come from the recipient's own or another donor. Transplantation of macrophages is a method of adoptive cell transfer that uses macrophages as the transplanted cells. This method can be used to treat diseases in which macrophages are missing or don't work right, like chronic granulomatous bone marrow deficiency, and lipid storage neurodegeneration. Transplanting macrophages can also be used to treat cancer by implanting genetically engineered or drug-treated macrophages into tumor sites to enhance their killing effect on tumor cells. CAR-Macrophage is an individualized form of adoptive transfer of macrophages that begins with the isolation of primary macrophages from a patient, which are then modified with the desired specific chimeric antigen receptor (CAR). The resulting modified macrophages are cryopreserved and will be infused back into the patient.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION