Introduction of Glycoengineering
In recent decades there has been an accelerated drive towards the increased development of protein-based drugs due to their great economic and clinical importance. Although proteins display multiple therapeutically favorable properties, their development and employment are often hindered as these routinely display suboptimum therapeutic efficacies due to intrinsic limitations in their physicochemical and pharmacological properties. As a result, there is great interest in the development and employment of molecular-level approaches to improve the therapeutic efficacy of protein drugs by engineering their physicochemical and pharmacological properties. A promising approach being employed involves the strategic manipulation of the protein surface glycosylation patterns through glycoengineering.
Applications of N-Glycanengineering
Avoidance of Aacroheterogeneity
Macroheterogeneity on recombinant glycoproteins arises from variations in glycosylation site occupancy. These differences glycosylation efficiency are dependent on the manufacturing system and protein intrinsic features. While all eukaryotic cells have an overall conserved machinery for N-glycosylation and display similar structural requirements for efficient N-glycosylation, there are minor differences in the utilization of glycosylation sites between species. Consequently, glycosylation sites may be skipped leading to underglycosylation of recombinant proteins or additional sites may be used leading to aberrant glycosylation. Protein intrinsic factors like the surrounding amino-acid sequence and secondary structure, the positioning of the consensus sequence within the polypeptide as well as the presence of other protein modifications contribute to N-glycosylation efficiency. Recognition of these glycoprotein-specific features depends on the presence and function of the different oligosaccharyltransferase (OST) subunits. Engineering of the OST complex is one possible way to overcome differences related to N-glycosylation site occupancy.
Reduction of Microheterogeneity
The commonly used expression systems for recombinant glycoproteins typically produce a mixture of different glycans on the same protein. This means that individual sites on the same protein can be furnished with glycans of high structural diversity. Microheterogeneity arises from variations in N-glycan processing which depends on numerous cellular factors including tissue/celltype-specific expression of processing enzymes, their concentrations and enzyme kinetic parameters, availability of the nucleotide sugar donors and the glycoprotein residence, and contact time in the reaction compartment. In addition, intrinsic protein structural properties can have a strong effect on site-specific N-glycan processing. The genetic inactivation or inhibition of competitive glycosyltransferases and glycoside hydrolases is a beneficial strategy to prevent unwanted glycan modification and reduce the complexity of glycosylation. At present, recombinant glycoproteins have been successfully produced in yeast or plants by homogeneous glycosylation to reduce microheterogeneity.
Elimination of Potentially Immunogenic Sugar Residues
The majority of the currently used expression systems for glycoprotein therapeutics produce N-glycans carrying nonhuman structures that can lead to potential side effects of the drugs. These non-human epitopes may elicit unwanted immune responses that neutralize the applied drug or even worse cause a hypersensitivity reaction. Beneficial properties can be engineered by the introduction of additional sugar residues that form novel recognition sites or mask binding sites for receptors involved in clearance. For instance, optimized pharmacokinetic behavior is achieved by increasing the sialic acid content of recombinant glycoproteins. Highly sialylated erythropoietin variants have been produced in mammalian cells as well as in nonmammalian systems including yeast, insect cells, and plants.
Optimization of Pharmacokinetics and Biological Activity
In recent years, the industry has put considerable effort into the development of novel glycoprotein therapeutics. Glycoengineering of cells for IgG1 expression has focused mainly on the elimination of core-fucose from the N-glycan at Asn297 in the Fc region of the heavy chain. The absence of core-fucose increases the affinity for FcgRIII receptor binding leading to improved antibody-dependent cellular cytotoxicity on natural killer cells. A similar glycosylation-dependent mechanism has an impact on antibody-dependent cellular phagocytosis by macrophages and influences the receptor-mediated effector function of virus-neutralizing antibodies. The ZMAPP antibody cocktail for the treatment of Ebola virus infections and reversion of the disease contains three plant-produced antibodies that lack core-fucose residues. In mice, the fucose-free monoclonal antibody 13F6 which is one of the ZMAPP components displayed enhanced potency against Ebola virus compared to 13F6 variants with core fucose. These examples clearly demonstrate the impact of glycosylation and highlight the potential of glyco-engineered mAbs for different applications in humans. As a consequence, the number of glyco-engineered mAbs approved for different treatments is expected to increase in the near future.
Glyco-engineered Therapeutic Glycoproteins
Apart from modulating effector functions, future glycosylation remodeling strategies will also focus on the engineering of mAbs for enhanced anti-inflammatory propertie. IgG glycoforms with high amounts of terminal sialic acid can display an increased affinity for lectin-type receptors and may represent another emerging field for the development of next-generation antibodies. Apart from the engineered antibody therapeutics, recombinant glycoprotein drugs with enhanced half-life, entirely humanized glycosylation, as well as different recombinant products for enzyme replacement therapy, are in the pipelines of companies.
Applications of O-glycanengineering
Remarkably, the contribution of distinct O-glycans to therapeutic properties is still not very well investigated. As a consequence, the specific targets for O-glycan engineering are less obvious than for N-glycan engineering. The development of systems capable of producing defined O-glycans is vital to improve our understanding of O-glycan function for glycoprotein therapeutics. IgA antibodies have, for example, high potential as a new class of drugs to combat infections or kill tumor cells. The engineering of O-glycan residues in the hinge region of IgA1 antibodies toward highly sialylated structures represents an interesting target to optimize the stability and pharmacokinetic behavior of recombinant IgA1 therapeutics.
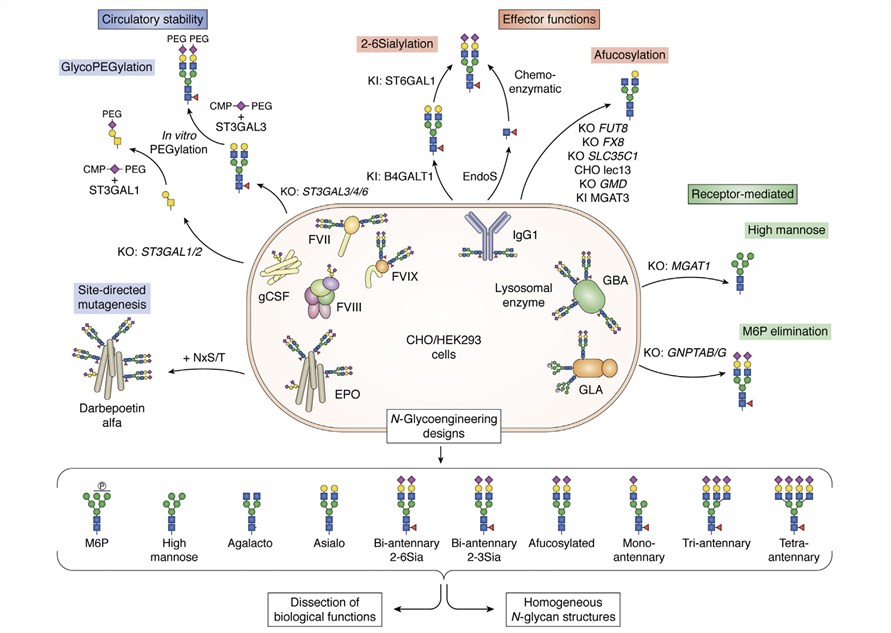 Fig.1 Glycoengineering strategies for recombinant glycoprotein therapeutics.1, 2
Fig.1 Glycoengineering strategies for recombinant glycoprotein therapeutics.1, 2
Services at Creative Biolabs
Natural proteins are an important source of therapeutic agents. While many of them have the potential to be used as highly effective medical treatments for a wide range of diseases, problems associated with poor biophysical and biological properties have limited their applications. Engineering proteins with reduced side-effects and/or improved biophysical and biological properties is therefore of great importance.
With proven experience in glycoscience, Creative Biolabs has accumulated substantial experience and expertise in glycoengineering and can provide customers with personalized Glycoengineering Service. Please contact us for more information.
Reference
-
Narimatsu, Yoshiki, et al. "Genetic glycoengineering in mammalian cells." Journal of Biological Chemistry 296 (2021). Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Glycoengineering strategies for recombinant glycoprotein therapeutics.1, 2
Fig.1 Glycoengineering strategies for recombinant glycoprotein therapeutics.1, 2

