Platforms for Recombinant Therapeutic Glycoprotein
Recombinant therapeutic proteins have been widely explored over the years for the treatment of a broad range of diseases. Currently, more than 250 biopharmaceutical products have received licenses in the US and European Union markets, being the vast majority of recombinant protein products. Compared with synthetic drugs, therapeutic recombinant proteins present some advantages: high specificity and complexity; well-tolerated and less immunogenic; and importantly, clinical development and regulatory approval could be faster than small-molecule drugs. Choosing the appropriate host for recombinant glycoproteins is a key step toward successful functional protein production
Since the 1980s, mammalian cells, mainly Chinese hamster ovary (CHO) cells, have been used for the production of glycosylated recombinant therapeutic proteins. Compared to human cell lines, CHO cells tend to add a small number of non-human glycans to recombinant proteins. If their quality is not well controlled, engineered glycoproteins produced by this expression system may cause an immune response. Despite this minor limitation, CHO cells offer multiple advantages. First, they can be cultured in large-scale bioreactors and their production rate of glycoproteins is much higher than that of human cells. Second, due to the natural differences in species, CHO cells are much less likely to transmit human pathogens. Because the advantages outweigh the disadvantages, CHO cells have become one of the most widely used mammalian cell expression systems for the production of glycoproteins.
Compared to mammalian cell lines, human cell lines are a powerful alternative to natural human protein production, improving their efficacy and reducing the risk of possible immunoreactions. HEK 293 is a transformed cell line derived from human embryonic kidney and is widely employed for recombinant protein production by transient expression. This cell line can grow easily in suspension under xenofree conditions, having, however, the tendency to form large aggregates. Four commercial products have been produced in this cell line. HT-1080 fibrosarcoma-derived cell is another human cell line that has been successfully used by the industry. Employing gene activation technology, three commercially approved therapeutic glycoproteins are available on the market and have been produced by the Shire. It is clear that human cells lines have advantages that favor their use as recombinant protein producers and with continuous research, investment and technology advances, human cells lines will be considered the gold standard for protein production with applications in different therapeutic areas.
The first biopharmaceutical approved, recombinant insulin, was produced in bacterial expression systems. Since then, it has been used to produce numerous commercially approved nonglycosylated therapeutic proteins, such as enzymes, cytokines and monoclonal antibodies, and has presented several limitations for recombinant glycoprotein production due to the lack of the enzymatic machinery required for mammalian-like glycosylation. For this reason, bacterial expression systems traditionally were not considered for the production of complex and glycosylated proteins. Although significant progress made over recent years regarding E. coli glycoengineering, the yields currently achieved are insufficient for commercial purposes.
The yeast-based expression system is considered very attractive and has been extensively used for the production of relevant proteins. Yeasts can be cultured in chemically defined media, grow rapidly achieving higher densities in a short period, present robust expression, have well-characterized glycosylation machinery and present the ability to scale up fermentation to industrial scale. Almost 20% of the biopharmaceuticals are being produced by S. cerevisiae, including insulin, hepatitis vaccines, and human serum albumin.
Similarly, to bacteria and yeast, plant cells can be easily cultured in basal culture medium and present a robust cell growth, being easily scaled up. Besides, they do not harbor human-trophic pathogens and endotoxins and are not subject to several disadvantages of recombinant glycoprotein produced in whole plants. This platform can synthesize complex protein and also glycoproteins, showing greater similarity to humans in terms of N-glycan structure. Accordingly, there is a recent and growing interest in using plant cells to produce biopharmaceuticals by biotechnology companies around the world.
Higher tolerance to osmolality and toxic metabolites, optimal protein expression at high levels, and no need for extensive culture handling are notable advantages related to the use of insect cells as a production platform for recombinant proteins. The insect cell-based platform has been successfully used to produce vaccine antigens and virus-like particles. Two FDA licensed vaccines and one autologous prostate-cancer therapy product have already been approved by regulatory agencies and many more are under clinical trial investigations.
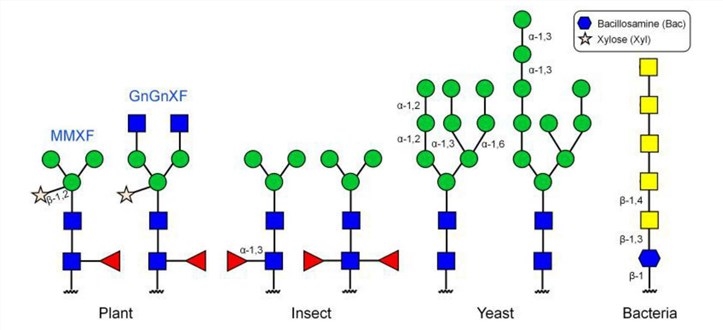 Fig.1 N-linked glycans on glycoproteins produced in different expression systems.1
Fig.1 N-linked glycans on glycoproteins produced in different expression systems.1
Services at Creative Biolabs
Choosing the appropriate host for recombinant therapeutic glycoproteins is a key step toward successful functional protein production. A plethora of aspects needs to be considered, including the host’s main characteristics, biochemical environment, production costs, capacity to process and translate the RNA transcript, safety, efficacy, and stability of the product.
Creative Biolabs has a series of recombinant protein platforms that can provide customized services and will greatly promote your progress in the drug development process. If you have any questions about recombinant therapeutic glycoprotein, please contact us for more information.
Reference
-
Ma, Bo, et al. "Protein glycoengineering: an approach for improving protein properties." Frontiers in Chemistry 8 (2020): 622. Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 N-linked glycans on glycoproteins produced in different expression systems.1
Fig.1 N-linked glycans on glycoproteins produced in different expression systems.1

