Research on Improving Therapeutic Glycoprtein Production
Glycosylation characteristics play a major role in modulating a protein’s stability, folding, targeting/trafficking, immunogenicity, biological activity, and especially circulatory half-life. Chinese hamster ovary (CHO) cells are widely used for the production of many commercial and clinical biopharmaceuticals due to their capacity to produce glycoforms that are, with exceptions, accepted by the human immune system. Alternative mammalian cell lines also used in the production of biopharmaceuticals include baby hamster kidney (BHK21), murine myeloma and hybridoma cell lines (NS0 and Sp2/0), and, to a lesser extent, human hosts cell lines such as human embryonic kidney (HEK293) and human retinal cells (PER. C6).
The glycosylation of biotherapeutics has been identified as a critical quality attribute because each biotherapeutic requires defining glycosylation characteristics to maintain consistent quality parameters. Thus, to tailor the glycosylation structures produced by CHO cells, many researchers have undertaken metabolic glycoengineering strategies to alter the final glycan profiles and distribution in CHO.
Combining Metabolic and Process Engineering Strategies to Improve the Production and Quality
The accumulation of metabolic waste is a major hurdle for recombinant protein production and quality in mammalian cell culture processes. Strategies to mitigate the accumulation of lactate and ammonia, the most detrimental waste metabolites, have been extensively studied. Even though no direct detrimental effect of lactate on product quality has been demonstrated yet, its accumulation has to be mitigated to favor cell viability and thus ensure optimal environmental conditions for recombinant protein quality. By improving glucose utilization efficiency, metabolic engineering strategies such as the overexpression of the PYC2 gene in mammalian cells help address the problem of lactate accumulation. Also, α-ketoglutarate, which is a TCA cycle intermediate, was shown to be effective at substituting glutamine by decreasing ammonia concentration by 4-fold and by increasing protein titer by 1.3-fold in the culture of a CHO cell line producing an Fc-fusion protein.
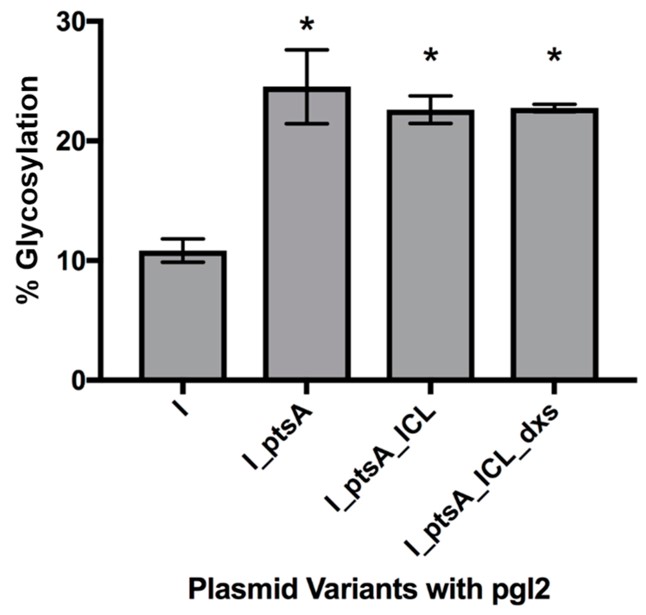 Fig.1 Improving glycosylation efficiency in Escherichia coli by metabolic engineering.1, 3
Fig.1 Improving glycosylation efficiency in Escherichia coli by metabolic engineering.1, 3
One can rely on genetic engineering strategies to develop cell lines with improved glycosylation capability, for instance, by overexpressing sialyltransferase and galactosyltransferase. Furthermore, optimization of culture conditions can be performed to rapidly and efficiently modulate protein production and quality. Some batch cultures exhibiting low cell densities showed enhanced cell-specific productivities.
Increasing the Efficiency of Glycoprotein Production in E. coli
The insert of foreign recombinant protein machinery that permits glycosylation into E. coli can have a significant effect on the host’s metabolism. By reducing the metabolic burden to the host, the efficiency of glycosylation has increased. One of the more successful approaches, was the use of inverse metabolic engineering, whereby a selection of genetic libraries of varying size from E. coli W3110 was transformed into the commonly used E. coli glycoprotein expression strain, CLM24. An iterative engineering strategy using quantitative proteomics and probabilistic metabolic modeling provided opportunities to discover variations in protein levels in E. coli cells overexpressing a recombinant glycoprotein. To optimize target protein glycosylation efficiency and total yield, accurate quantification and identification of the correctly glycosylated target protein are essential.
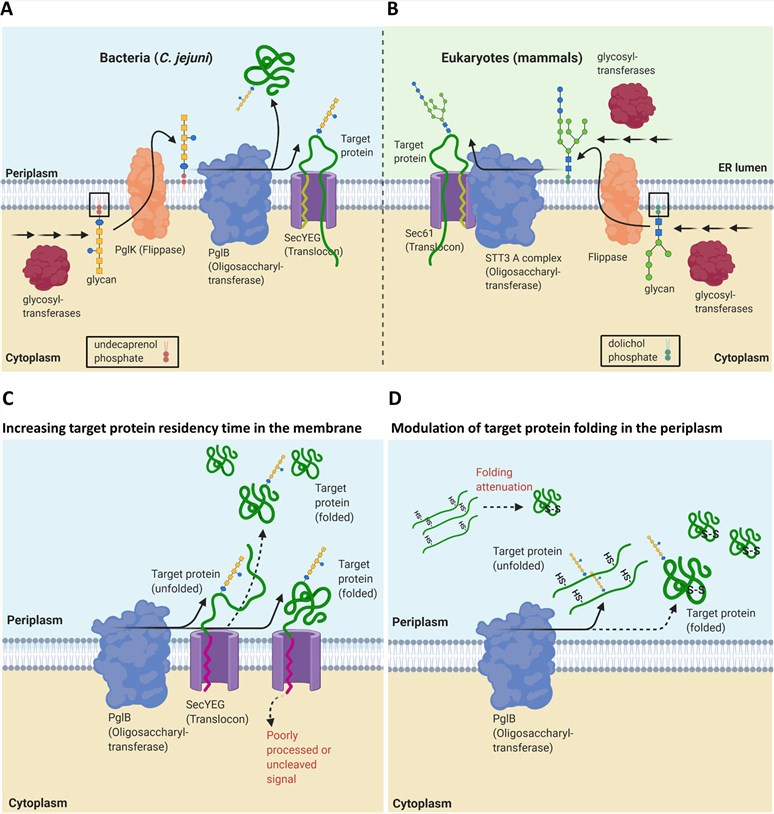 Fig. 2 Native N-linked protein glycosylation pathway and E. coli glycoengineering.2, 3
Fig. 2 Native N-linked protein glycosylation pathway and E. coli glycoengineering.2, 3
Creative Biolabs strives to produce high-potency therapeutic glycoprotein. We offer the most popular, state-of-the-art expression systems for our clients to choose from. All of our experts at Creative Biolabs are here to guide you through making the best decisions to enhance the competitive edge of your product or project. We are proud to offer our clients value-added services to enhance overall product quality. These services include the product testing carried out by our highly trained chemists and technicians. Please feel free to contact us for more details of your interested services or technologies.
References
-
Strutton, Benjamin, et al. "Engineering pathways in central carbon metabolism help to increase glycan production and improve N-type glycosylation of recombinant proteins in E. coli." Bioengineering 6.1 (2019): 27.
-
Pratama, Fenryco, Dennis Linton, and Neil Dixon. "Genetic and process engineering strategies for enhanced recombinant N-glycoprotein production in bacteria." Microbial cell factories 20 (2021): 1-25.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Improving glycosylation efficiency in Escherichia coli by metabolic engineering.1, 3
Fig.1 Improving glycosylation efficiency in Escherichia coli by metabolic engineering.1, 3
 Fig. 2 Native N-linked protein glycosylation pathway and E. coli glycoengineering.2, 3
Fig. 2 Native N-linked protein glycosylation pathway and E. coli glycoengineering.2, 3

