Introduction of Therapeutic Glycoprtein
The biopharmaceutical market is growing with a compounded annual growth rate (CAGR) of 16% above the pharma market (CAGR, 8%). This growth is based on the uniqueness of biopharmaceuticals that have revolutionized the treatment of many diseases where no other therapeutic approaches have been completely successful. While the enzymatic activity of biopharmaceuticals is determined by the protein structure, for several therapeutic proteins, glycosylation plays an essential role in pharmacokinetics, pharmacodistribution, protection from proteolytic degradation, solubility, receptor binding and, for monoclonal antibodies, enhancement of effector function.
Glycosylation of Therapeutic Proteins
The hyperglycosylated derivative of erythropoietin, darbepoetin, is generated by glycoengineering and contains additional N-linked glycosylation sites. As the sialic acid-containing glucan content of erythropoietin is directly proportional to the serum half-life and in vivo bioactivity, the two additional glycosylation sites in darbepoetin increase serum half-life threefold and enhance biological activity. Isoforms of follicle-stimulating hormone (FSH) with high sialic acid content at the two N-linked glycosylation sites show reduced renal clearance and increased biological activity. For the derivative FSH1208, a consensus N-linked glycosylation sequence extension was added to one of the subunits of FSH, which was efficiently glycosylated and increased the serum half-life fourfold. Reduced renal clearance of the protein resulted in increased half-life and thereby biological activity was enhanced. The stability of therapeutic proteins is of high importance for storage, shipment and therapeutic response. In this respect, glycosylation can protect the therapeutic protein from proteolytic degradation and increase its temperature stability.
Glycosylation of Monoclonal Antibodies
Monoclonal antibodies are presently the fastest growing class of biopharmaceuticals, with a CAGR for their development of 36%. Their fast development from first identification to market is based on the availability of extracellular targets of therapeutic relevance identified by genomic and proteomic techniques that have been applied to various diseases (especially in oncology and immunology). Monoclonal antibodies have many activities in addition to neutralizing antigens. For example, the Fc domain of the monoclonal antibody is responsible for complement activation. Interestingly, there is a linear increase of in vitro complement activation with increasing terminal galactosylation of the carbohydrate moiety in the Fc domain. In addition, antibody-dependent cell cytotoxicity (ADCC) depends partially on the specific N-glycosylation of Asn in the Fc domain of both heavy chains of the monoclonal antibody. The absence of fucose, or the presence of a bisecting N-acetylglucosamine in the carbohydrate structure of the monoclonal antibody, increases the potency of ADCC and suggests the need for lower therapeutic doses in vivo.
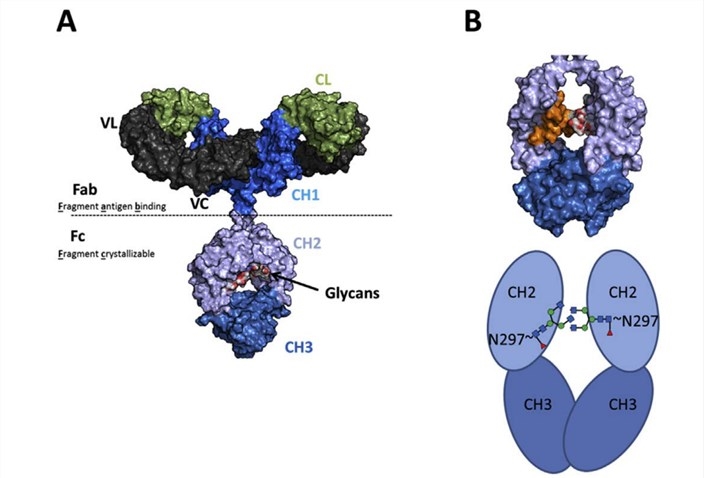 Fig.1 Domain structure of several therapeutic antibody formats. (Cymer, 2018)
Fig.1 Domain structure of several therapeutic antibody formats. (Cymer, 2018)
FDA Approved Antitumor Therapeutic Monoclonal Antibodies
The therapeutic market for monoclonal antibodies (MAbs) has grown exponentially since 2000. By 2020, the global market for monoclonal antibodies has exceeded US$125 billion. Antitumor monoclonal antibody therapy is an important branch of tumor immunotherapy. Since the first antitumor therapeutic Mab was approved to treat non-Hodgkin lymphoma by targeting at CD20 on B cells in 1997, so far there have been nearly 30 antitumor MAbs approved by FDA. As targeted drugs, antitumor therapeutic MAbs usually target highly expressed surface antigens of tumor cells, like CD20, EGFR, and PD-L1, which could play a role in tumorigenesis, angiogenesis, and immunosuppression. Due to the high efficacy, research and development of antitumor MAbs have competed fiercely in pharmaceutical enterprises. To discover novel targets, and to explore new functions of existing targets and interaction between multiple targets are the major tasks of biotechnology and bioengineering in the future.
Over the past twenty years, therapeutic proteins have rapidly become the leading product within the biopharmaceutical market. With first-in-class technologies and experienced scientists, Creative Biolabs has successfully developed versatile glycoprotein production platforms. Based on these cutting-edge platforms, we can provide our worldwide clients with the largest and most diverse portfolio of standard or custom therapeutic glycoprotein services. If you have any questions about therapeutic glycoprotein, please do not hesitate to contact us immediately, we will provide you with professional advice.
Reference
-
Cymer, F.; et al. Therapeutic monoclonal antibody N-glycosylation - structure, function and therapeutic potential. Biologicals: journal of the International Association of Biological Standardization. 2018, 52: 1-11.
For Research Use Only.
Related Services:
- Introduction of Glycoengineering
- Platforms for Recombinant Therapeutic Glycoprotein
- Research on Improving Therapeutic Glycoprotein Production
- Therapeutic Glycoprotein Drug Applications Status
- Glycoprotein Pharmaceuticals: Scientific and Regulatory Considerations
- Bio-Therapeutic Glycoproteins Market

 Fig.1 Domain structure of several therapeutic antibody formats. (Cymer, 2018)
Fig.1 Domain structure of several therapeutic antibody formats. (Cymer, 2018)
