With evolving protein therapeutic platforms, newer biopharmaceuticals have been developed as important pharmaceuticals for the treatment of multiple diseases. The safety and efficacy of biological candidates are critical to their clinical success. However, the proteinaceous nature of many drug candidates can lead to an immunogenic response in the patient. As a pioneer company offering immunogenicity assessment services, Creative Biolabs is capable of providing high-quality immunogenicity analysis services of drug candidates for the treatment of autoimmune disease.
It has been reported that a number of factors could influence the immunogenicity of biopharmaceuticals. In terms of the drug, the presence of nonhuman sequences or novel epitopes generated by amino acid substitution designed to enhance stability, or novel epitopes created at the junction of fusion proteins may contribute to the appearance of immunogenicity response. In addition, molecular structure, changes in glycosylation, can also influence the immunogenicity of a biopharmaceutical. Once the immune system is activated, the efficacy of the biotherapeutic declines as the system attempts to clear the antigen. The immune response itself affects product safety as cytokine release can be toxic. For biotherapeutic development, avoiding this response is vital for success.
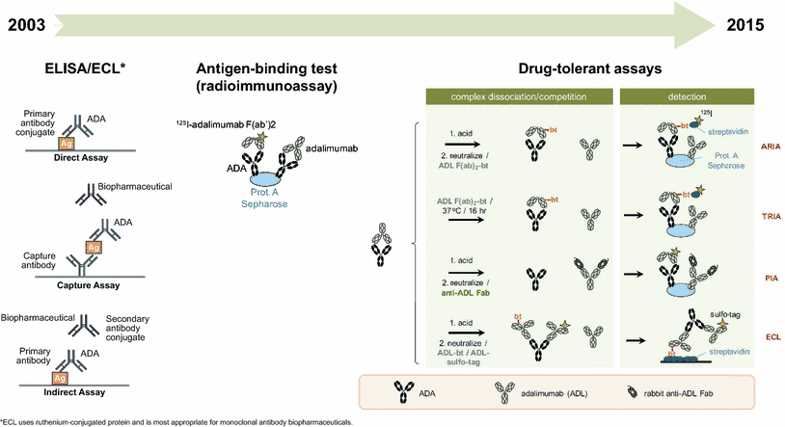 Fig.1 Evolution of immunogenicity assays for biopharmaceuticals and assessment of anti-adalimumab antibodies. (Pineda, 2016)
Fig.1 Evolution of immunogenicity assays for biopharmaceuticals and assessment of anti-adalimumab antibodies. (Pineda, 2016)
Animal testing has been used in an effort to assess the potential immunogenicity of biotherapeutic and protein candidates. Even though helpful to researchers, it is often not a good indicator of what the human immune response will be to the biological candidate. Currently, in silico and in vitro assays are the most widely used methods to assess potential immunogenicity of biotherapeutics. Based on sequence information and structural bioinformatics, in silico assay provides a predictive tool to help determine the likelihood of peptide/HLA binding, a condition necessary for T cell activation. While, in vitro approach utilizes a high throughput, multi-parameter approach to analyze T and B cell responses to the drug candidate. At Creative Biolabs, we offer a full package of assays for potential immunogenicity prediction, which including but not limited to Flow Cytometry, ELISPOT, and ELISA.
Creative Biolabs is committed to offering tailor-designed immunogenicity assessment services to meet our global customers’ needs in a fast, reliable and affordable way. If you are interested in our service to support your development program, please contact us by sending us your detailed request to e-mail. Our scientists will get in touch with you as soon as possible.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.