Natural killer (natural killer, NK) cells are lymphocytes of the innate immune system, which play an important role in preventing virus infection and inhibiting the development of cancer. NK cells account for 10%-15% of the total number of human peripheral blood lymphocytes. Because of the dense intracellular cytolytic granules expressed in the cells, they are also called large granular lymphocytes. When some abnormal tumor cells or virus infected cells are encountered, NK cells spontaneously activate and release the granular contents, perforin and granzymes, to the target cells. Perforin and granzymes can induce apoptosis of target cells. NK cells need to recognize and adhere to abnormal cells one-to-one in order to exert cytotoxicity. Therefore, NK cells are considered to be particularly important for the elimination of unicellular tumors, especially leukemia, lymphoma and metastatic tumor cells. Although NK cells were originally named for their ability to produce cytotoxicity, NK cells can also produce many cytokines and chemokines, especially interferon-γ (IFN-γ), tumor necrosis factor α (TNFα) and GM-CSF. In addition to acting directly on tumor cells and viral infected cells, these cytokines can also promote the differentiation, activation and/or recruitment of other immune cells.
Classification of NK Cells
NK cells express many cell surface molecules, such as CD56, CD16, CD57, CD161, etc., but these surface markers are not unique to NK cells, that only have relative specificity. Lymphoid cells expressing CD56, CD16, CD3, TCR and BCR are usually regarded as NK cells, so some scholars use surface markers to classify NK cells. Scientists have found that CD56 is a specific marker of NK cell differentiation, and the expression of CD16 is closely related to the killing activity of NK cells. Therefore, human NK cells can be divided into two types according to their immunophenotypes and functions: CD56dim and CD56bright.
CD56dim NK cells, which make up 90% of the total NK cell population in peripheral blood, express low affinity receptors for the constant region of immunoglobulin G, Fc γ RIIIa (CD16). Functionally, they have high cytotoxic activity.
CD56bright NK cells, which account for 10% of the total NK cell population in peripheral blood. They are mainly involved in the production of cytokines.
NK Cell-mediated Immune Responses
NK cells mediate their immunomodulatory effects through two key effector functions. First, NK cells are cytotoxic lymphocytes that directly cleave cells that have undergone malignant transformation or are infected with viruses or other intracellular pathogens. The cytolytic function of NK cells can be initiated through a variety of processes, including degranulation and death receptor ligation, which are essential for the removal of diseased and dysfunctional cells. Secondly, NK cells can produce a variety of inflammatory cytokines in response to activated receptor stimulation and inflammatory cytokine-induced activation signals. These effector functions are an important part of the immune response and the main mechanism of protective immunity mediated by NK cells.
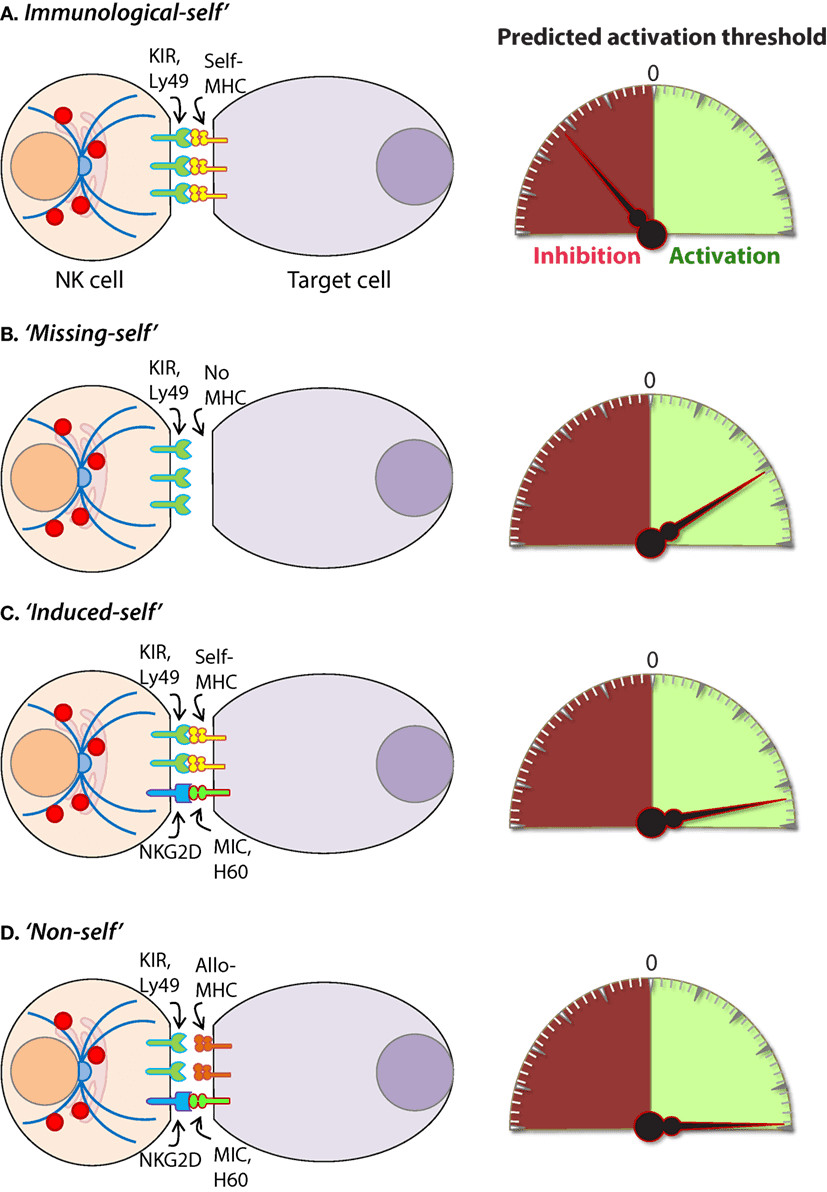 Fig.1 Mechanisms of target cell recognition by natural killer (NK) cells.2,3
Fig.1 Mechanisms of target cell recognition by natural killer (NK) cells.2,3
The function of NK cells is controlled by a variety of receptors expressed on the cell surface. These receptors are essentially inhibitory or activating. The inhibitory receptor family consists of killer immunoglobulin-like receptors (KIR) or Ig-like receptors (CD158), the C type lectin receptors (CD94-NKG2A) and leukocyte inhibitory receptors (LIR1, LAIR-1). Activating receptors are the natural cytotoxicity receptors (NKp46, NKp44), C type lectin receptors (NKG2D, CD94-NKG2C), and Ig-like receptors (2B4). In addition to a series of activated receptors, specific NK cells typically express 2-4 inhibitory receptors. Because different NK cells express different combinations of inhibitory or activating receptors, there is considerable heterogeneity in the NK cell population. Therefore, NK cells are considered to have the ability to respond to a variety of stimuli and to participate in the immune response under various pathological conditions.
The molecular mechanism of regulating NK cytotoxicity has been well described and can be divided into three main processes: (1) target cell recognition, (2) target cell contact and immunological synapse (IS) formation, and (3) target cell death induced by NK cells.
The cytotoxicity of NK cells is strictly regulated by the balance between activating and inhibition signals. The inhibitory NK cell receptors (NKRs) recognize self-MHC class I molecules, thus preventing the activation of NK cells. This explains self-tolerance and prevents host cells from killing. It has been found that NK cells are activated when they encounter cells that lack self-MHC class I molecules. This is the so-called "missing-self" hypothesis. In addition, NK cells can distinguish between normal host cells and infected or abnormal cells by recognizing MHC class I molecules. Virally infected cells and tumor cells usually down-regulate the expression of MHC class I, thereby escaping the recognition of cytotoxic T lymphocytes (CTL), but this makes them vulnerable to NK cells. In this case, the activating receptors are no longer inhibited, they induce effective stimulation signals, so the balance tends to be beneficial to the activation of NK cells. This condition is often referred to as induced-self recognition.
Once the target is recognized by NK cells, their cytotoxic ability is mainly mediated by two main pathways. A membrane-disrupting protein, perforin, and a family of structurally related serine proteases, granzymes, are secreted by exocytosis, which jointly induce apoptosis of the target cell. In the second pathway, a caspase-dependent apoptosis takes place involving the association of death receptors (e.g. Fas/CD95) on target cells with their equivalent ligands such as FasL, and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on NK cells, resulting in caspase-dependent apoptosis. Antibody-dependent cytotoxic (ADCC) can also be a mechanism by which NK cells kill tumor cells because they express the low-affinity Fc receptor for IgG, FcγRIII (CD16).
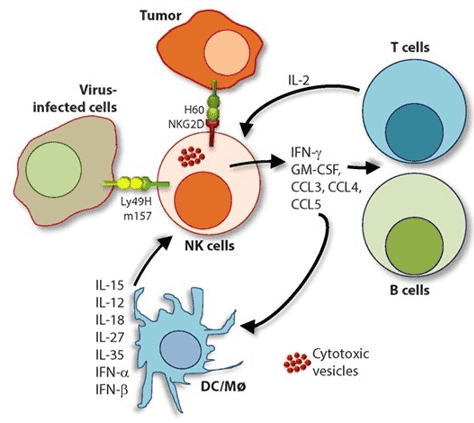 Fig.2 Role of a "third signal" in natural killer (NK) cell activation.2,3
Fig.2 Role of a "third signal" in natural killer (NK) cell activation.2,3
NK cells are effective producers of pro-inflammatory and immunosuppressive cytokines. However, the release of inflammatory cytokines is different from that of cytotoxic granules, and NK cells use activation-induced signaling components to regulate these two functions differently. Although NK cells can produce a wide range of cytokines according to the inflammatory environment, they mainly produce Th1-type cytokines when they respond to tumor ligands and intracellular pathogens. These Th1-type cytokines include IFN-γ, TNF and granulocyte/monocyte colony-stimulating factor (GM-CSF), which promote the activation of T cells and other innate immune mediators such as DC cells, macrophages and neutrophils. NK cells also produce chemotactic cytokines (chemokines), including CCL3 (MIP-1α), CCl4 (MIP-1β), CCL5 (RANTES), XCL1 (lymphotoxin) and CXCL8 (IL-8), which attract effector lymphocytes and myeloid cells to inflamed tissues.
Transcriptional activation of cytolytic molecules and inflammatory cytokines is a highly regulated process mediated by various transcriptional regulators in NK cells. Many of these transcription factors are lineage defining and activated in the early stages of NK cell development. Cytokine-induced transcription factors activation occurs in response to IL-12 and IL-2 + IL-15 signals respectively, such as signal Transducers and Activators of Transcription 4 and 5 (STAT4, STAT5). NK cell receptors also initiate the inflammatory transcriptional process upon activation. These include c-Fos and c-Jun heterodimers, AP-1, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and nuclear factor of activated T cells which bind promotor regions and promote inflammatory cytokine gene transcription.
NK Cell-based Immunotherapies
Based on the important role of NK cells in the immune system, many small molecular drugs targeting NK cells and NK cell-based immunotherapies have been developed for the treatment of refractory diseases, including viral infection and cancer. Immunotherapy based on NK cells mainly utilizes the NK cell-mediated immune response mechanism and the role of other immune cells, such as T cells, B cells, dendritic cells (DCs) and macrophages. Currently developed NK cell-based immunotherapies mainly include adoptive NK cell therapy, CAR-based NK cell immunotherapy, NK cell-targeted vaccine and NK cell immunotherapy targeting CSCs.
Specific monoclonal antibodies (mAbs) that recognize tumor cell surface antigens are used in cancer treatment. These therapeutic mAbs target and attack tumor cells through a variety of mechanisms, including guiding toxic molecules to target cells, inhibiting the proliferation of target cells, blocking inhibitory signals against immune cells, and guiding immune cells to kill targets through antibody-dependent cellular cytotoxicity (ADCC). Due to the essential roles in the immune system, NK cell-expressed receptors, ligands, and cytokines have been regarded as attractive targets for therapeutic antibody development.
As a kind of adoptive cell transfer (ACT) therapy, adoptive NK cell therapy aims to remove, enrich, repair or expand the biological function of immune cells by modified or unmodified autologous NK cells (obtained from patients) and allogeneic NK cells (obtained from healthy donors). In order to increase the number of functional NK cells, the required cells can come from a variety of sources, including PBMC, BM, cord blood, human embryonic stem cells, induced pluripotent stem cells and human NK cell lines.
Another biotechnology-based method to derive an efficient cytotoxic NK cell is engineering of chimeric antigen receptor (CAR)-expressing NK cells, which represents an excellent source of off-the-shelf cellular therapy for patients with cancer. Similar to CAR-modified T cells, the first generation of CAR-engineered human NK cells mainly consists of an extracellular antigen recognition domain (e.g., single-chain variable fragment, scFv, of a mAb), fused to a transmembrane domain, and intracellular signaling domains, which provide a signal to activate NK cells.
NK cells exhibit the characteristics of adaptive immune cells, including their ability to specifically recognize microbial antigens and their potential to develop into long-lived memory cells to prevent subsequent infection. These findings suggest that new vaccine strategies should be developed to promote the induction of long-lived, pathogen-specific memory NK cells that could contribute to prevention or control of infection.
A tumor contains a very small subset of heterogeneous populations of quiescent cells, known as CSCs. CSCs have been suggested to be responsible for tumor repopulation, metastasis, and tumor relapse, and resistance to conventional cytoreductive therapies. In vitro studies have claimed that CSCs of several solid cancers express ligands for activating NK cells receptors. Due to the fact that immune cells do not need tumor cell targets to be actively cycling and mediated their effects, targeting CSCs with stem-like properties in sustaining tumor progression via immunotherapy is a promising method to eradicate CSCs. Moreover, the combination of NK cell–based immunotherapy with cytoreductive therapies results in enhanced T and NK cell-mediated killing of CSCs due to increased expression of the stress-derived activator ligands on surviving CSCs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION