Cancer immunotherapy, which works by activating the body's own immune system, has become a very important research direction in the treatment of cancer. In recent years, the success of anti-tumor therapy with antibodies and cell-based immunotherapy has become a landmark event of tumor therapy. As innate immune cells, natural killer (NK) cells are unique and can play a key role in cancer immune surveillance. NK cells do not need to be sensitized in advance to eliminate a variety of abnormal or compressed cells, and even give priority to kill stem cell-like cells or cancer stem cells. After forming immune synapses with target cells, NK cells release pre-formed cytolytic granules, including perforin and granzyme, to induce cell lysis. Several studies have successfully used the adoptive transfer of NK cells to target a variety of tumors, especially hematological malignancies.
However, tumor cells use a variety of strategies to delay, change or even stop anti-tumor immunity, resulting in the failure of tumor cell control. The anti-tumor response of NK cells also faces many limitations. First, the lack of NK cells' ability to reach tumor tissue limits their use as solid tumor therapy. This is a common problem with cellular immunotherapy strategies. Secondly, changes in NK cell-activated receptors and their ligands in tumors may lead to reduced response to therapy and tumor progression. For example, high levels of NKG2D ligands are detected in the early stages of colorectal cancer, but their expression decreases as the disease progresses. Third, tumor microenvironment (TME) is still a major obstacle to the effectiveness of adoptive transfer of NK cells. For example, tumor infiltrating immune cells embedded in extracellular matrix may meddle in the activation of NK cells by secreting immunosuppressive cytokines or interfering with the expression of receptors. In addition, in TEM, TGF-β is considered to be the main inhibitory cytokine of NK cells, which limits the number and anti-metastasis function of NK cells. Other factors, such as prostaglandin E2, adenosine or indoleamine 2, 3 talk dioxygenase, can also block the cytotoxic activity of NK cells by, respectively, inducing myeloid-derived suppressor cells, binding to adenosine A2A receptors expressed on NK cells or catalyzing tryptophan-producing L-kynurenine to inhibit the expression of NKp46 and NKG2D on NK cells. It is also found that the cytotoxicity of NK cells is inhibited by activated platelets in malignant milieu through a series of mechanisms, including the shed and transfer of their MHC class I molecules to tumor cells; undermining NK cell effector function via platelet-derived TGF-β or shielding tumor cells from NK cell attack.
Regulation of NK Cell Responses
Because NK cells do not need to contact or recognize specific tumor antigens in advance, their activity must be highly regulated to limit their response to healthy tissues. The first is to inhibit the expression of receptors. The receptors encoded by these strains are divided into three main types: 1) killer immunoglobulin-like receptor (KIR), 2) c-lectin, NKG2A/CD94, and 3) leukocyte immunoglobulin-like receptor (LILR). The ligands for these inhibitory receptors are commonly expressed major histocompatibility class-I molecules (MHC-1). When MHC-1 molecules bind to inhibitory receptors expressed in NK cells, they provide inhibitory signals to NK cells, preventing their activation, degranulation blocking and cytokine production. Because healthy cells express normal levels of MHC-1 molecules, this mechanism can be used as a form of tolerance of NK cells and protect healthy tissue from NK cells killing. During the development of NK cells, inhibitory receptor ligation with cognate MHC-I can also be used as a mechanism to "educate" NK cells to respond to MHC-I deficient cells. However, this is not the only mechanism that regulates the activity of NK cells.
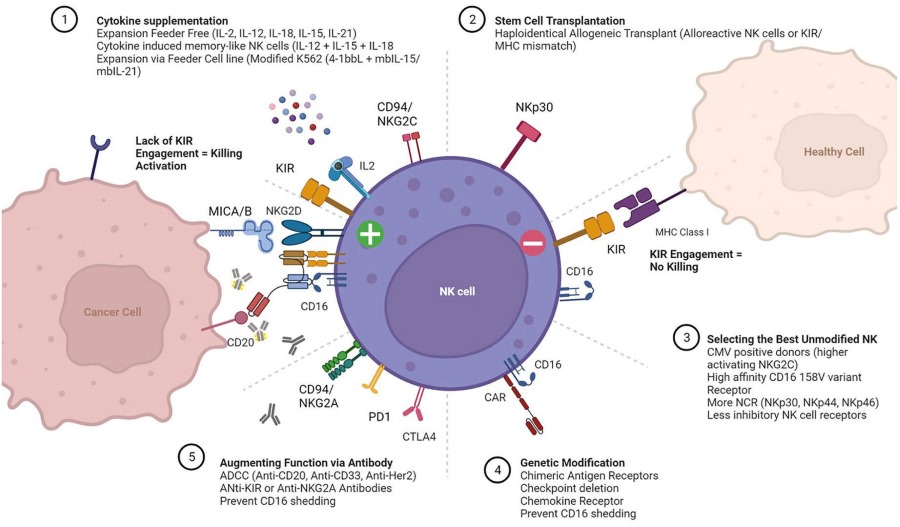 Fig.1 Regulation of NK cell responses.1
Fig.1 Regulation of NK cell responses.1
NK cells kill tumor target cells through a variety of mechanisms. The destruction of tumor cells by receptor-mediated cytotoxicity is one of the most important abilities of NK cells. Natural killer cells express a variety of receptors encoded by various lines, such as the c-type lectin homodimer NKG2D, which binds to stress-induced ligands (e.g. ULBP, MICA/MICB) typically expressed on tumor cells. After ligation, NK cells degranulated and released perforin and granzyme to induce apoptosis of target cells. NK cell degranulation can also be triggered by a process called antibody dependent cell-mediated cytotoxicity (ADCC). This process depends on the existence of tumor-specific antibodies that bind to tumor surface antigens. The Fc portion of these antibodies binds to the low-affinity Fc receptor CD16 on NK cells and triggers degranulation. NK cells can also mediate the killing of target cells through death receptor-mediated apoptosis. NK cells can express FasL, a member of tumor necrosis (TNF) family, or TNF-related apoptosis inducing ligand (TRAIL), which can interact with their respective ligands Fas and TRAIL receptor (TRAILR) expressed on tumor cells. NK cells can directly lyse tumor-transformed cells, and they can also act as a bridge between innate and adaptive immune response to enhance the recognition and destruction of adaptive immune cells to tumor. This is achieved through the production and secretion of cytokines such as IFN-γ, which can restrict tumor angiogenesis and increase the expression of MHC-II on tumor cells and antigen-presenting cells, thereby enhancing the adaptive immune response.
Despite the mechanisms by which NK cells can recognize and destroy tumor-transformed cells, over the process of tumor progression, malignant cells will still form the opposite mechanism. Through these mechanisms, tumors can disrupt or alter immune responses, including those from NK cells. By down-regulating the expression of adhesion molecules, activating ligands or costimulatory molecules of activated receptors, and up-regulating the expression of MHC-1 molecules or releasing soluble activated ligands, tumor cells can escape the response of NK cells. These cells can also affect the function of NK cells by secreting immunosuppressive factors. NK cell defects observed in cancer patients include decreased expression of activated receptors, decreased expression of intracellular signal molecules and overexpression of inhibitory receptors. Therefore, these NK cells showed decreased cytotoxic and cytokine secreting functionality as well as reduced proliferation.
Sources of NK Cells for Adoptive Transfer
The preliminary study of adoptive transfer of NK cells aims to improve the anti-tumor response of autologous NK cells by using cytokines to enhance the function and proliferation of NK cells. Stimulation of NK cells with cytokines results in increased expression of adhesion molecules, cytokine induced activated receptors (NKp44), perforin, granzyme and FasL, TRAIL, as well as proliferation and cytokine production. Because of the unacceptable toxicity and minimal benefit obtained from NK stimulation trails with high dose of IL-2 in vivo, culturing human peripheral blood (PB) NK cells with cytokines in vitro seems to be an alternative strategy. However, IL-2 incubated "lymphokine-activated killer" (LAK) cells also failed to produce optimal results. The limitations leading to this failure were identified as the unintended proliferation of Treg cells, which eventually leads to the NK cell suppression, and the inhibitory effects of self-HLA molecules highly expressed on malignant cells.
To eliminate these limitations, attempts have been made to use allogeneic NK cells. NK cells usually come from PB. After following PB apheresis from normal donors, CD3 depletion was performed to prevent GvHD. Sometimes the product is also depleted of CD19, designed to prevent passenger lymphocyte syndrome and Epstein-Barr virus (EBV) reactivation. In another attempt to improve the purity of NK cells, CD56-positive selection can be performed after CD3 depletion, but there is a risk of reducing the yield of NK cells. In the end, the cell product is either administered immediately, incubated with cytokines, or undergoes ex vivo expansion. However, the latter is usually necessary because NK cells account for only 5%-20% of PB mononuclear cells. Autologous feeder cells and/or genetically modified allogeneic feeder cells are used in order to provide the survival, proliferation, and activation signals required for NK cell expansion. For this purpose, irradiated cell populations have been used, such as PB monocytes, T cells, EBV transformed lymphoblasts and K562 cells. Newly developed feeder-free protocol has the potential to achieve a higher purity ratio of NK cells. However, they suffer from donor dependent variabilities in cell yield and purity, which have to be improved. Although there are various protocols for the selection and expansion of NK cells, only a few of them meet the strict requirements of good manufacturing practice (GMP), which is a prerequisite for clinical use.
Although patients and related donors are still the main sources of adoptive transfer of NK cells, stem cell-derived NK cells are still a positive focus of research. Differentiation of mature, functional NK cells can be achieved through the culture of bone-marrow or umbilical cord blood derived CD34 + hematopoietic stem cells with IL-2/IL-15 along with allogeneic feeder cells and various growth factors such as stem cell factor and FLT-3L. These NK cells showed immature phenotype, similar to CD56bright NK cells, but showed strong cytotoxicity and cytokine production. However, some of these cells express KIR and CD16. Other studies, tested whether IPS cells can differentiate into hematopoietic stem cells and then into NK cells, have shown that this method is feasible. These NK cells seem to be more like CD56dim populations because they mediate ADCC and have an effective anti-tumor response in xenogeneic mice. In addition, IPS derived NK cells can be used as a renewable source of therapeutic cells and can be genetically modified to express chimeric antigen receptors (CARs) against specific tumor antigens to enhance their efficacy.
Malignant NK cell lines are another field of clinical research. The potential benefit of these cell lines is that they cannot be inhibited by the recipient HLA due to the low expression of KIR's or KIR-HLA mismatch in the recipient. NKL, KHYG-1 and NKG cell lines have good anti-tumor activity in vitro. The function and proliferation ability of NKL cell line is similar to that of CD56dimCD16+ NK cell subsets. Their ability to mediate ADCC and natural cytotoxicity makes them the main candidates for adoptive immunotherapy. The main advantage of using the NK cell lines is that it is easy to expand and maintain NK cell lines for immunotherapy under good manufacturing practice conditions. Their function can also be enhanced by cytokines. Therefore, adoptive transfer of NK cell lines may be proved to be an effective alternative method to isolate or transfer autologous or allogeneic NK cells for tumor.
Recently, the NK cell population has been identified, which is expanded in response to cytomegalovirus (CMV) infection and has anti-CMV activity and powerful function against tumor targets. Identified by their expression of the activation receptor NKG2C/CD94 and the maturation marker CD57, these NK cells have been shown to expand after CMV reactivation in transplant recipients. Studies have shown that these cells are transplantable and are effective IFN-γ producers when exposed to K562 cells or through CD16 signaling. The enhanced function of these cells makes them an area of concern for future use in tumor immunotherapy.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION