Purification Methods of Glycoprotein
Overview of Glycoprotein
Protein glycosylation is one of the most studied post-translational modifications due to its crucial role in the quality control and regulation of protein folding, stability, functional efficiency, and trafficking. In addition to the major N- and O-linked glycosylations, protein phosphorylation, C-mannosylation, and GPI anchor glycosylation (or glypiation) have been identified. Such protein modifications can considerably alter the secondary structure of the protein, which may change its activity or interaction with other molecules and, consequently, affect diverse biological processes, such as gene expression, cell adhesion, signal transduction, and immune responses. Thus, aberrant glycosylation may be related to the progression of diseases such as cancer, neurodegenerative disorders, and other genetic abnormalities.
Purification Methods of Glycoprotein
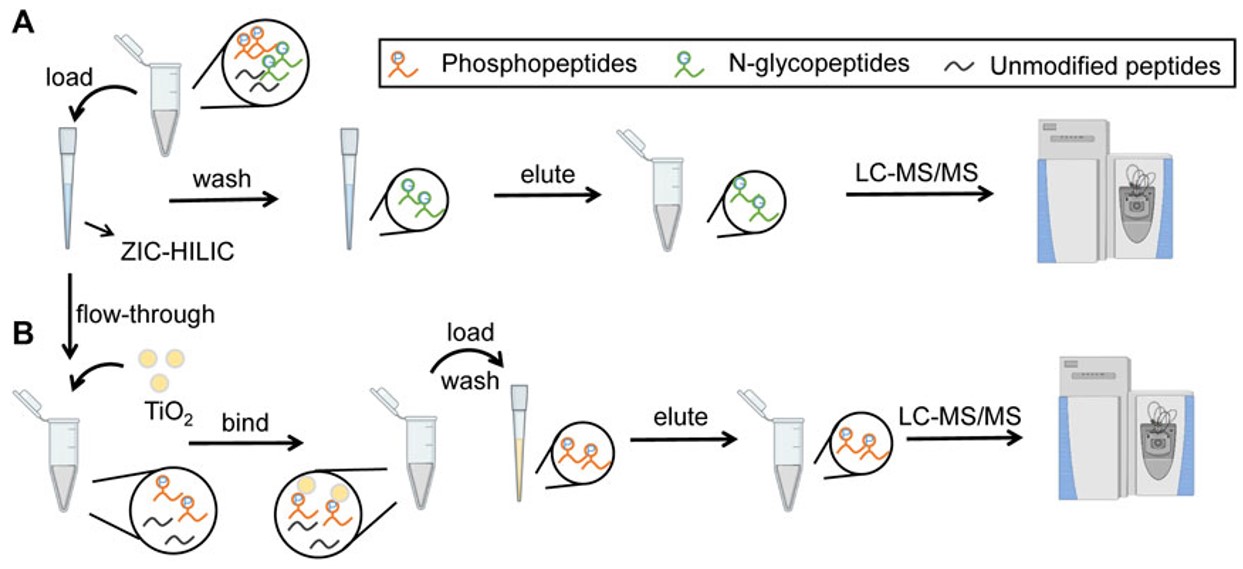 Fig.1 Workflow of glycopeptide enrichment.1, 3
Fig.1 Workflow of glycopeptide enrichment.1, 3
-
Normal-phase HPLC (NP-HPLC)
Normal-phase HPLC (NP-HPLC) uses a water-non-miscible normal mobile phase, and the retention mechanism is predominantly surface adsorption; in contrast, HILIC uses a water-miscible organic solvent (typically acetonitrile) with retention via a partitioning mechanism, which is analogous to liquid-liquid solvent extraction; the stationary phase is the solid support. One of the HILIC's major advantages is its easy interface with electrospray ionization mass spectrometry (ESI-MS) through the use of volatile organic solvents and buffers for simultaneous peptide identification, glycosylation-site detection, and glycan structural analysis. HILIC is a simple and excellent approach for enriching and fractionating complex glycopeptide mixtures and it is a promising tool for identifying novel glycosylation sites and glycoproteins in biological samples.
Due to their selective recognition of specific glycan moieties on glycoconjugates, lectins have been widely utilized as affinity probes to efficiently detect glycans in complex biological fluids. Many lectins have been employed in advanced biomedical applications, including cancer treatment, fluorescent visualizations for molecular imaging, sensor arrays for cancer cell detection, and drug delivery. In lectin affinity chromatography, lectins are the recognition elements that capture either intact glycoproteins or pre-digested glycopeptides. Efficient separation depends on the type of lectin and experimental conditions used. In general, separation may be easy through varying the conditions, such as buffer composition, pH, and in certain special cases, the type of the metal ion. Bound glycoconjugates are eluted from lectins by disrupting the hydrogen bond interaction between the ligand and sugar at a low pH with buffers such as 0.1% trifluoroacetic acid (TFA) or glycine HCl upon washing out the unbound materials. Alternatively, certain molecules, such as specific mono- or disaccharides, are utilized to compete with the glycotope for lectin binding.
-
Chelation/coordination chemistry
Metal ion affinity chromatography is generally a useful tool for separating biomolecules with unique ion- or ligand-exchange properties, such as phosphorylated and glycosylated peptides. In this category, the critical interaction is bond formation through coordination between the unoccupied orbital on the metal ion (on the chromatographic solid support) and the spare electron pair from the oxygen atom (in carboxylate or hydroxyl groups) or the nitrogen atom (in amine groups) on the glycan. Moreover, electrostatic interaction between negatively charged glycans and positively charged ions on the solid support surface may also influence affinity, as demonstrated by the easily enriched sialic acid-containing glycans through TiO2 chromatography.
Unlike lectin affinity methods, enrichment through covalent bonding is a universal method because the binding is based on carbohydrate cis-diol group reactivity without discrimination between different glycan structures. Among chemical glycan modification methods, covalent bond formation between glycopeptides and functionalized solid supports is primarily based on two strategies: boronic acid and hydrazide chemistry. The primary distinction between the two reactions is reversibility; while boronic acid chemistry is easily reversible without altering the glycan structure, hydrazide chemistry requires irreversible and destructive glycan oxidation to generate aldehyde groups.
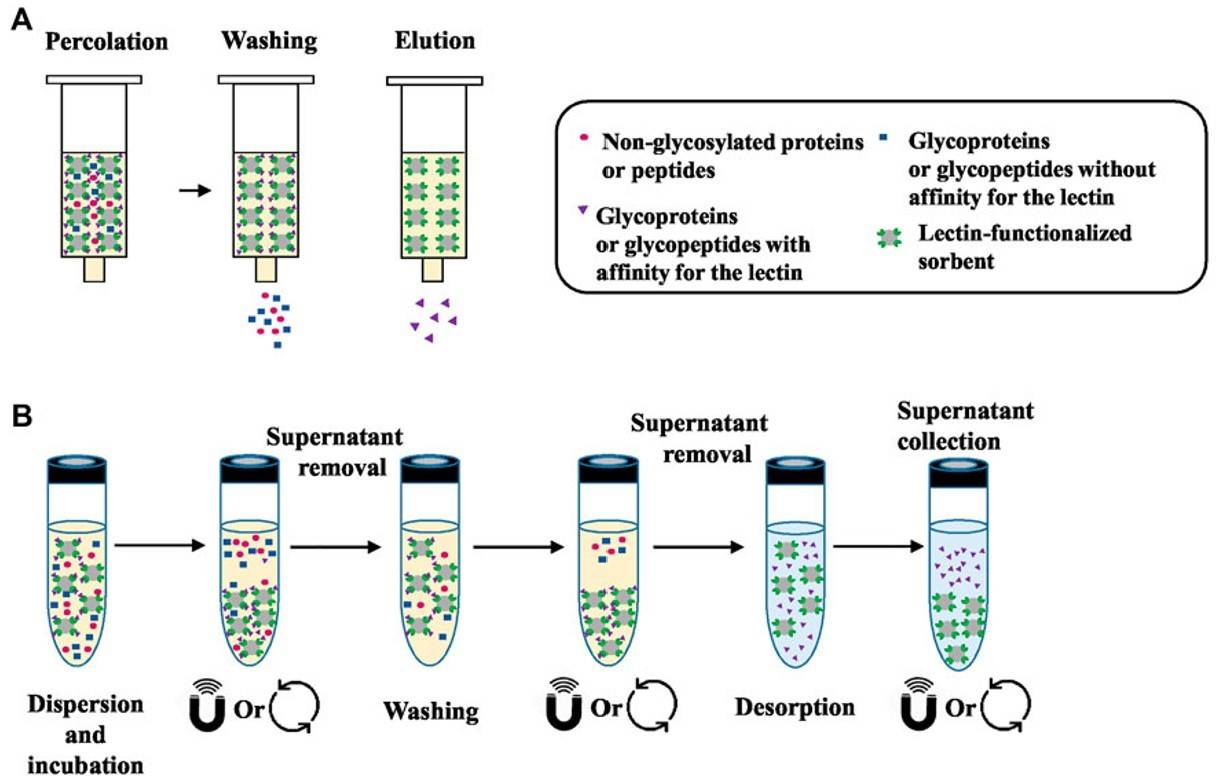 Fig.2 Lectin-based glycoprotein purification process.2, 3
Fig.2 Lectin-based glycoprotein purification process.2, 3
Services at Creative Biolabs
Creative Biolabs has been focusing on glycoprotein researches over years and thus accumulated rich experience. We are confident in providing high-quality glycoprotein-based services to global customers including but not limited to:
If you are looking for a partner in glycoprotein research or you have any other questions about our services, please feel free to contact us for more information.
References
-
Du, Zhuokun, et al. "A new strategy for high-efficient tandem enrichment and simultaneous profiling of N-glycopeptides and phosphopeptides in lung cancer tissue." Frontiers in Molecular Biosciences 9 (2022): 923363.
-
Goumenou, Anastasia, Nathalie Delaunay, and Valérie Pichon. "Recent advances in lectin-based affinity sorbents for protein glycosylation studies." Frontiers in Molecular Biosciences 8 (2021): 746822.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Workflow of glycopeptide enrichment.1, 3
Fig.1 Workflow of glycopeptide enrichment.1, 3
 Fig.2 Lectin-based glycoprotein purification process.2, 3
Fig.2 Lectin-based glycoprotein purification process.2, 3

