Research Progress of Glycoprotein Vaccine
Glycan chains on glycoproteins play a role in exogenous antigen recognition and immune system activation. Primary examples in vaccine design have shown good levels of carbohydrate-specific antibody generation when raised using extracted or fully synthetic capsular polysaccharide glycans covalently coupled to a protein carrier. Conjugate vaccines, in which polysaccharide antigens are covalently linked to carrier proteins, are one of the most effective and safest vaccines against bacterial and viral pathogens. With the development of protein engineering, glycoprotein vaccines have shown large application potential due to high bio-compatibility. Creative Biolabs is a world-leading company that can provide the best Glycoprotein-based Vaccine Development service for global customers in the field of protein research. Based on our established support platforms including MS, LC-ESI-MS, MALDI-TOF MS, HPLC, HPAEC-PAD, and Flow Cytometry, we have successfully completed many related vaccines discovery projects and can provide the best services to customers all over the world. 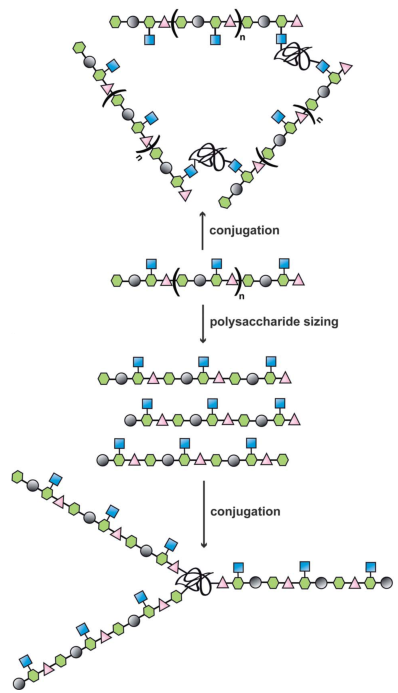 Fig.1 Illustration of approatches in glycoprotein
Fig.1 Illustration of approatches in glycoprotein
vaccine design.1, 2
Introduction of Glycoprotein Vaccine
Bacterial and virus polysaccharides are high molecular weight molecules acting as T-cell independent antigens. Unlike proteins, glycans cannot be processed by antigen-presenting cells, which leads to the result that they rather trigger B-cell responses by cross-linking the B-cell receptor, without any MHCII CD4+ T-cell interaction. As a result, polysaccharides induce B-cells to differentiate into plasma cells and further lead to the secretion of low-affinity antibodies, but without the formation of a persistent memory B-cell pool.
However, when glycan chains are covalently linked to proteins, the resulting conjugates are taken into the endosomes in association with MHCII and presented to CD4+ T-cells. Peptide/MHCII-activated T-cells release cytokines to stimulate B-cell maturation to memory cells while large amounts of high-affinity IgG antibodies can be produced. Consequently, immunization with recombined glycoproteins induces longer and stronger protection against pathogens compared with the same carbohydrate antigen. Hence, the general structure of a glycoconjugate vaccine consists of a carbohydrate B-cell epitope and a protein or peptide providing the T-helper epitope to ensure T-dependent memory response.
Development of Glycoprotein Vaccine
Oligosaccharide synthesis and site-specific protein modification methodologies have seen immense advances and are now available. With these, it is now possible to design and synthesize chemically defined glycoconjugate vaccines with varying carbohydrate densities, conformations, and shapes, which would rely on an easier physicochemical characterization in comparison to current carbohydrate-based vaccines, and hopefully lead to the selection of candidates with enhanced safety and efficacy.
Moreover, some research suggested that recombined glycan conjugate vaccines can be produced in vivo using the general glycosylation pathway of E. coli. This in vivo biosynthesis of glycoprotein vaccines is more convenient and rapid compared with chemical methods.
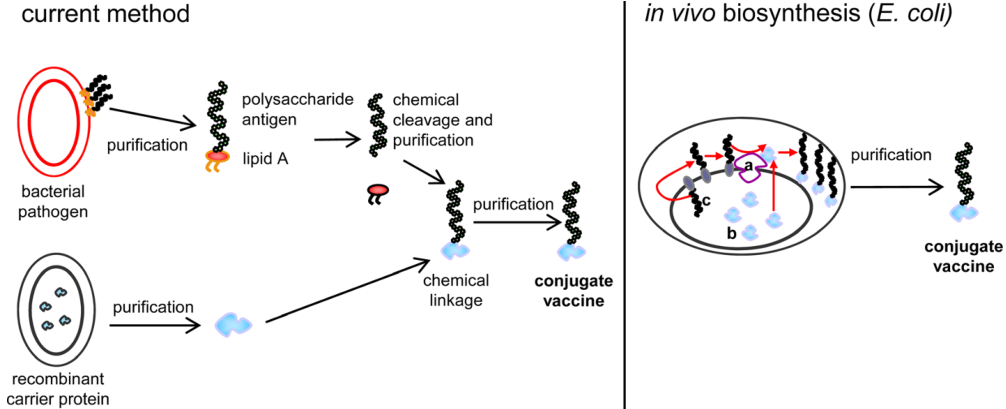 Fig.2 Current method for the production of conjugate vaccines and in vivo biosynthesis.3, 4
Fig.2 Current method for the production of conjugate vaccines and in vivo biosynthesis.3, 4
With the development of novel technologies, the safety and efficacy of glycovaccines have been further improved and occupied a position in vaccine research. Based on a comprehensive platform, Creative Biolabs launched mature and one-stop Glyco-based Vaccine Development services including Glycoprotein-based Vaccine Development and Glycopeptide-based Cancer Vaccine Development. For more detail on our services or interest in vaccine development, please feel free to contact us.
References
-
Adamo, Roberto, et al. "Synthetically defined glycoprotein vaccines: current status and future directions." Chemical science 4.8 (2013): 2995-3008.
-
Under Open Access license CC BY 3.0, without modification.
-
Ihssen, Julian, et al. "Production of glycoprotein vaccines in Escherichia coli." Microbial cell factories 9 (2010): 1-13.
-
Under Open Access license CC BY 2.0, without modification.
For Research Use Only.
Resources

 Fig.1 Illustration of approatches in glycoprotein
Fig.1 Illustration of approatches in glycoprotein Fig.2 Current method for the production of conjugate vaccines and in vivo biosynthesis.3, 4
Fig.2 Current method for the production of conjugate vaccines and in vivo biosynthesis.3, 4

