Ryanodine receptor 1 (RYR-1) is a protein found mainly in skeletal muscle encoded by the RYR1 gene in humans. It is also known as Skeletal muscle ryanodine receptor, Skeletal muscle calcium release channel, Skeletal muscle-type ryanodine receptor and Type 1 ryanodine receptor. RYR1 is related to the dihydropyridine receptor (l-type calcium channel) in the myofibrillary membrane of t-tubules, which is opened in response to depolarization, suggesting effectively that RYR1 channel is opened in response to cell depolarization.
| Basic Information of RYR1 | |
| Protein Name | Ryanodine receptor 1 |
| Gene Name | RYR1 |
| Aliases | Skeletal muscle calcium release channel, Skeletal muscle ryanodine receptor, Skeletal muscle-type ryanodine receptor, Type 1 ryanodine receptor |
| Organism | Homo sapiens (Human) |
| UniProt ID | P21817 |
| Transmembrane Times | 6 |
| Length (aa) | 5038 |
| Sequence | MGDAEGEDEVQFLRTDDEVVLQCSATVLKEQLKLCLAAEGFGNRLCFLEPTSNAQNVPPDLAICCFVLEQSLSVRALQEMLANTVEAGVESSQGGGHRTLLYGHAILLRHAHSRMYLSCLTTSRSMTDKLAFDVGLQEDATGEACWWTMHPASKQRSEGEKVRVGDDIILVSVSSERYLHLSTASGELQVDASFMQTLWNMNPICSRCEEGFVTGGHVLRLFHGHMDECLTISPADSDDQRRLVYYEGGAVCTHARSLWRLEPLRISWSGSHLRWGQPLRVRHVTTGQYLALTEDQGLVVVDASKAHTKATSFCFRISKEKLDVAPKRDVEGMGPPEIKYGESLCFVQHVASGLWLTYAAPDPKALRLGVLKKKAMLHQEGHMDDALSLTRCQQEESQAARMIHSTNGLYNQFIKSLDSFSGKPRGSGPPAGTALPIEGVILSLQDLIIYFEPPSEDLQHEEKQSKLRSLRNRQSLFQEEGMLSMVLNCIDRLNVYTTAAHFAEFAGEEAAESWKEIVNLLYELLASLIRGNRSNCALFSTNLDWLVSKLDRLEASSGILEVLYCVLIESPEVLNIIQENHIKSIISLLDKHGRNHKVLDVLCSLCVCNGVAVRSNQDLITENLLPGRELLLQTNLINYVTSIRPNIFVGRAEGTTQYSKWYFEVMVDEVTPFLTAQATHLRVGWALTEGYTPYPGAGEGWGGNGVGDDLYSYGFDGLHLWTGHVARPVTSPGQHLLAPEDVISCCLDLSVPSISFRINGCPVQGVFESFNLDGLFFPVVSFSAGVKVRFLLGGRHGEFKFLPPPGYAPCHEAVLPRERLHLEPIKEYRREGPRGPHLVGPSRCLSHTDFVPCPVDTVQIVLPPHLERIREKLAENIHELWALTRIEQGWTYGPVRDDNKRLHPCLVDFHSLPEPERNYNLQMSGETLKTLLALGCHVGMADEKAEDNLKKTKLPKTYMMSNGYKPAPLDLSHVRLTPAQTTLVDRLAENGHNVWARDRVGQGWSYSAVQDIPARRNPRLVPYRLLDEATKRSNRDSLCQAVRTLLGYGYNIEPPDQEPSQVENQSRCDRVRIFRAEKSYTVQSGRWYFEFEAVTTGEMRVGWARPELRPDVELGADELAYVFNGHRGQRWHLGSEPFGRPWQPGDVVGCMIDLTENTIIFTLNGEVLMSDSGSETAFREIEIGDGFLPVCSLGPGQVGHLNLGQDVSSLRFFAICGLQEGFEPFAINMQRPVTTWFSKGLPQFEPVPLEHPHYEVSRVDGTVDTPPCLRLTHRTWGSQNSLVEMLFLRLSLPVQFHQHFRCTAGATPLAPPGLQPPAEDEARAAEPDPDYENLRRSAGGWSEAENGKEGTAKEGAPGGTPQAGGEAQPARAENEKDATTEKNKKRGFLFKAKKVAMMTQPPATPTLPRLPHDVVPADNRDDPEIILNTTTYYYSVRVFAGQEPSCVWAGWVTPDYHQHDMSFDLSKVRVVTVTMGDEQGNVHSSLKCSNCYMVWGGDFVSPGQQGRISHTDLVIGCLVDLATGLMTFTANGKESNTFFQVEPNTKLFPAVFVLPTHQNVIQFELGKQKNIMPLSAAMFQSERKNPAPQCPPRLEMQMLMPVSWSRMPNHFLQVETRRAGERLGWAVQCQEPLTMMALHIPEENRCMDILELSERLDLQRFHSHTLRLYRAVCALGNNRVAHALCSHVDQAQLLHALEDAHLPGPLRAGYYDLLISIHLESACRSRRSMLSEYIVPLTPETRAITLFPPGRSTENGHPRHGLPGVGVTTSLRPPHHFSPPCFVAALPAAGAAEAPARLSPAIPLEALRDKALRMLGEAVRDGGQHARDPVGGSVEFQFVPVLKLVSTLLVMGIFGDEDVKQILKMIEPEVFTEEEEEEDEEEEGEEEDEEEKEEDEEETAQEKEDEEKEEEEAAEGEKEEGLEEGLLQMKLPESVKLQMCHLLEYFCDQELQHRVESLAAFAERYVDKLQANQRSRYGLLIKAFSMTAAETARRTREFRSPPQEQINMLLQFKDGTDEEDCPLPEEIRQDLLDFHQDLLAHCGIQLDGEEEEPEEETTLGSRLMSLLEKVRLVKKKEEKPEEERSAEESKPRSLQELVSHMVVRWAQEDFVQSPELVRAMFSLLHRQYDGLGELLRALPRAYTISPSSVEDTMSLLECLGQIRSLLIVQMGPQEENLMIQSIGNIMNNKVFYQHPNLMRALGMHETVMEVMVNVLGGGESKEIRFPKMVTSCCRFLCYFCRISRQNQRSMFDHLSYLLENSGIGLGMQGSTPLDVAAASVIDNNELALALQEQDLEKVVSYLAGCGLQSCPMLVAKGYPDIGWNPCGGERYLDFLRFAVFVNGESVEENANVVVRLLIRKPECFGPALRGEGGSGLLAAIEEAIRISEDPARDGPGIRRDRRREHFGEEPPEENRVHLGHAIMSFYAALIDLLGRCAPEMHLIQAGKGEALRIRAILRSLVPLEDLVGIISLPLQIPTLGKDGALVQPKMSASFVPDHKASMVLFLDRVYGIENQDFLLHVLDVGFLPDMRAAASLDTATFSTTEMALALNRYLCLAVLPLITKCAPLFAGTEHRAIMVDSMLHTVYRLSRGRSLTKAQRDVIEDCLMSLCRYIRPSMLQHLLRRLVFDVPILNEFAKMPLKLLTNHYERCWKYYCLPTGWANFGVTSEEELHLTRKLFWGIFDSLAHKKYDPELYRMAMPCLCAIAGALPPDYVDASYSSKAEKKATVDAEGNFDPRPVETLNVIIPEKLDSFINKFAEYTHEKWAFDKIQNNWSYGENIDEELKTHPMLRPYKTFSEKDKEIYRWPIKESLKAMIAWEWTIEKAREGEEEKTEKKKTRKISQSAQTYDPREGYNPQPPDLSAVTLSRELQAMAEQLAENYHNTWGRKKKQELEAKGGGTHPLLVPYDTLTAKEKARDREKAQELLKFLQMNGYAVTRGLKDMELDSSSIEKRFAFGFLQQLLRWMDISQEFIAHLEAVVSSGRVEKSPHEQEIKFFAKILLPLINQYFTNHCLYFLSTPAKVLGSGGHASNKEKEMITSLFCKLAALVRHRVSLFGTDAPAVVNCLHILARSLDARTVMKSGPEIVKAGLRSFFESASEDIEKMVENLRLGKVSQARTQVKGVGQNLTYTTVALLPVLTTLFQHIAQHQFGDDVILDDVQVSCYRTLCSIYSLGTTKNTYVEKLRPALGECLARLAAAMPVAFLEPQLNEYNACSVYTTKSPRERAILGLPNSVEEMCPDIPVLERLMADIGGLAESGARYTEMPHVIEITLPMLCSYLPRWWERGPEAPPSALPAGAPPPCTAVTSDHLNSLLGNILRIIVNNLGIDEASWMKRLAVFAQPIVSRARPELLQSHFIPTIGRLRKRAGKVVSEEEQLRLEAKAEAQEGELLVRDEFSVLCRDLYALYPLLIRYVDNNRAQWLTEPNPSAEELFRMVGEIFIYWSKSHNFKREEQNFVVQNEINNMSFLTADNKSKMAKAGDIQSGGSDQERTKKKRRGDRYSVQTSLIVATLKKMLPIGLNMCAPTDQDLITLAKTRYALKDTDEEVREFLHNNLHLQGKVEGSPSLRWQMALYRGVPGREEDADDPEKIVRRVQEVSAVLYYLDQTEHPYKSKKAVWHKLLSKQRRRAVVACFRMTPLYNLPTHRACNMFLESYKAAWILTEDHSFEDRMIDDLSKAGEQEEEEEEVEEKKPDPLHQLVLHFSRTALTEKSKLDEDYLYMAYADIMAKSCHLEEGGENGEAEEEVEVSFEEKQMEKQRLLYQQARLHTRGAAEMVLQMISACKGETGAMVSSTLKLGISILNGGNAEVQQKMLDYLKDKKEVGFFQSIQALMQTCSVLDLNAFERQNKAEGLGMVNEDGTVINRQNGEKVMADDEFTQDLFRFLQLLCEGHNNDFQNYLRTQTGNTTTINIIICTVDYLLRLQESISDFYWYYSGKDVIEEQGKRNFSKAMSVAKQVFNSLTEYIQGPCTGNQQSLAHSRLWDAVVGFLHVFAHMMMKLAQDSSQIELLKELLDLQKDMVVMLLSLLEGNVVNGMIARQMVDMLVESSSNVEMILKFFDMFLKLKDIVGSEAFQDYVTDPRGLISKKDFQKAMDSQKQFSGPEIQFLLSCSEADENEMINCEEFANRFQEPARDIGFNVAVLLTNLSEHVPHDPRLHNFLELAESILEYFRPYLGRIEIMGASRRIERIYFEISETNRAQWEMPQVKESKRQFIFDVVNEGGEAEKMELFVSFCEDTIFEMQIAAQISEPEGEPETDEDEGAGAAEAGAEGAEEGAAGLEGTAATAAAGATARVVAAAGRALRGLSYRSLRRRVRRLRRLTAREAATAVAALLWAAVTRAGAAGAGAAAGALGLLWGSLFGGGLVEGAKKVTVTELLAGMPDPTSDEVHGEQPAGPGGDADGEGASEGAGDAAEGAGDEEEAVHEAGPGGADGAVAVTDGGPFRPEGAGGLGDMGDTTPAEPPTPEGSPILKRKLGVDGVEEELPPEPEPEPEPELEPEKADAENGEKEEVPEPTPEPPKKQAPPSPPPKKEEAGGEFWGELEVQRVKFLNYLSRNFYTLRFLALFLAFAINFILLFYKVSDSPPGEDDMEGSAAGDVSGAGSGGSSGWGLGAGEEAEGDEDENMVYYFLEESTGYMEPALRCLSLLHTLVAFLCIIGYNCLKVPLVIFKREKELARKLEFDGLYITEQPEDDDVKGQWDRLVLNTPSFPSNYWDKFVKRKVLDKHGDIYGRERIAELLGMDLATLEITAHNERKPNPPPGLLTWLMSIDVKYQIWKFGVIFTDNSFLYLGWYMVMSLLGHYNNFFFAAHLLDIAMGVKTLRTILSSVTHNGKQLVMTVGLLAVVVYLYTVVAFNFFRKFYNKSEDEDEPDMKCDDMMTCYLFHMYVGVRAGGGIGDEIEDPAGDEYELYRVVFDITFFFFVIVILLAIIQGLIIDAFGELRDQQEQVKEDMETKCFICGIGSDYFDTTPHGFETHTLEEHNLANYMFFLMYLINKDETEHTGQESYVWKMYQERCWDFFPAGDCFRKQYEDQLS |
RYR1 acts as a calcium release channel in the sarcoplasmic reticulum and a link between the sarcoplasmic reticulum and the transverse tube. RYR1 plays a signal role in the formation of embryonic bone. There is a correlation between the expression of ryr1-mediated Ca²⁺ signaling and the multi-molecular expression involved in the key myogenic signaling pathway. RYR1 is mechanically linked to neuromuscular junctions for the release of calcium-induced biological processes by calcium. Mutations in the RYR1 gene are associated with malignant hyperthermia, central nuclear disease, external eye myopathy, Samaritan myopathy, and benign congenital myopathy. In addition, splicing of different forms of transcripts is also demonstrated. Dantrolene may be the only known drug that is effective in cases of malignant hyperthermia.
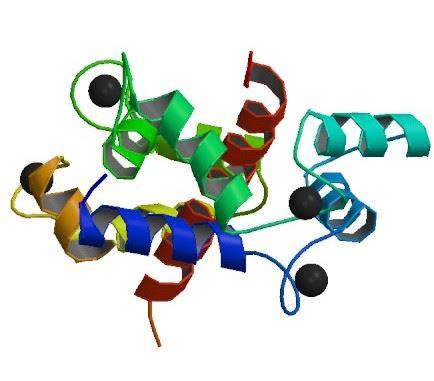 Fig.1 The structure of Ryanodine receptor 1.
Fig.1 The structure of Ryanodine receptor 1.
Recent studies have described the near-atomic structure of RyRs in mammalian bone and heart muscle, providing a structural basis for the regulation of multiple effectors of RyRs.
The authors envision that this system will have an impact on the molecular determinants of disease-associated RyR mutations and RyR channel modulation.
These results suggest that changes in disease severity may be affected by post-translational modification or hyperactivity caused by RyR1 dysfunction.
These results indicate that in the presence of all three activating ligands, high-resolution remodeling of the open and closed states of RyR1 was obtained from the same sample, and the conformational changes associated with gating were analyzed.
The authors conclude that Ca²⁺ activated channel remote variant gate control. The new gate control mechanism and the new ion selectivity mechanism of RyR1 are explained by the in-depth structural analysis.
To obtain the soluble and functional target protein, the versatile Magic™ membrane protein production platform in Creative Biolabs enables many flexible options, from which you can always find a better match for your particular project. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-RYR1 antibody development services.
Creative Biolabs' skillful scientists are glad to leverage our expertise and advanced technologies to help you with the member protein research. If you are interested, please feel free to contact us for more details.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.