One of the most important aspects of the manufacturing of cell-based therapy products is the definition and measurement of cell characteristics. This can be broken down into identity, potency, purity, and safety that must all be considered during product development. With the help of our well-established technologies and years of experience, Creative Biolabs can provide phenotypic, genetic, and functional assessments of your MSC population of interest using various techniques and tools. We adapt to your requirements by providing either individual service modules or a fully comprehensive service package.
Due to the population heterogeneity of MSC cultures resulting from factors such as different tissue derivations, diverse isolation protocols, various growth conditions, a common guideline for MSC preparations is required to be defined to characterize MSC populations. The basic characteristics and minimal criteria of MSCs were declared in 2006 by the International Society for Cellular Therapy (ISCT). Since then, additional markers have been identified and widely accepted as reliable MSC surface markers, such as STRO-1, CD271, CD348, CD200, and CD105. Studies have also found that functional and phenotypic differences may exist across tissue sources, culture conditions, and extent of ex-vivo expansion. Thus, tissue-specific expression of different surface markers should be considered when characterizing MSCs derived from different sources.
We offer a comprehensive analysis of MSCs to help biotechnology companies or researchers better understand the characteristics of these important cell types. Our services include the following:
Flow cytometry is at present the gold standard tool for studying the immunophenotype of ex-vivo expanded MSC. In this process, MSCs are stained with a panel of antibodies against different surface antigens and then analyzed by flow cytometry. Both single-color flow cytometry and multi-color flow cytometry can be used. Besides, when equipped with a cell sorter, it can be used to isolate specific populations.
Confirmation of tri-lineage differentiation (osteogenic, chondrogenic and adipogenic differentiation potential) of MSCs provides excellent evidence for the verification of MSC identity. This can be analyzed in vitro under different differentiation culture conditions and monitored by specific staining and molecular differentiation markers. Typically, osteogenic differentiation can be measured by staining for alkaline phosphatase and calcium deposition, adipogenic differentiation by staining with Oil Red O, and chondrogenic differentiation by staining with Alcian Blue or Toluidine Blue. Besides, molecular expression levels of differentiation markers are analyzed by qRT-PCR.
MSCs have long been known to be immunomodulatory and this property has been at the forefront of clinical studies. MSCs can suppress the activation of the immune system by regulating cells of the lymphoid immune and innate immune systems. Creative Biolabs is able to perform a comprehensive profiling of MSC immunomodulation in vitro using different tools and techniques.
Although MSCs are considered as genetically stable, it is necessary to conduct STR analysis due to the risk of genomic and structural chromosome instability in the long-term culture process. At Creative Biolabs, analysis of STR profiling can be carried out on different passages to authenticate the cell cultures.
Karyotype analysis is performed to identify the cells with chromosomal and chromatid fragments, deletions and translocations, thereby assessing chromosome structure instability. Methods such as GTG staining are widely used for karyotype abnormality analysis at different passages.
Our MSCs characterization services provide a comprehensive and detailed analysis of MSCs to support biotechnology companies and researchers in their research and development efforts. Our team of experts is dedicated to providing high-quality data and insights to advance your understanding of these important cell types.
Creative Biolabs is dedicated to providing unrivaled services and quality to scientists and companies working in the field of cell therapy and regenerative medicine. The combined expertise and techniques provide our customers with unique capabilities and flexible support. If you are interested in any of our services, please don't hesitate to contact us for more information.
Below are the findings presented in the article related to the characterization of MSCs.
Adipose-derived MSC is a simple and readily available source of MSC isolation. Usman Rashid, et al. identified a promising source of canine MSC isolation and differentiation. They isolated and processed adipose tissue from the subcutaneous inguinal (SC), perirenal (PR), omentum magnum (OM), and infrapatellar fat pad (IPFP) to isolate MSC and evaluated MSC proliferation/metabolism, surface marker expression, in vitro differentiation potential, and quantitative reverse transcription PCR.
They evaluated the cell surface antigen expression of MSCs from four adipose sources by flow cytometry and immunofluorescence. The results showed that all MSCs from adipose tissue sources showed positive expression of CD73 (NT5E), CD90 (THY1), CD105 (ENDOGLIN), and very low expression of CD45.
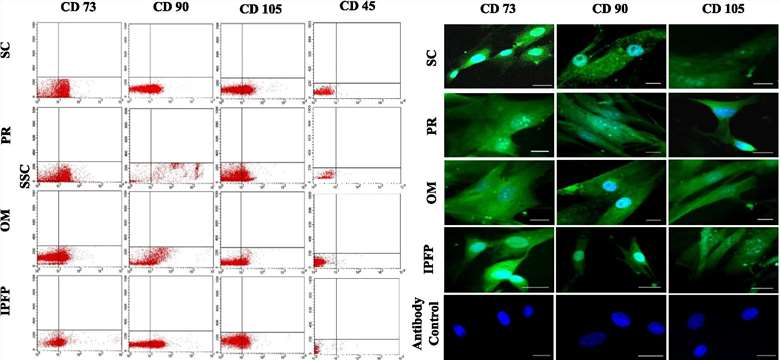 Fig. 1 Flow cytometric expression of CD73, CD90, CD105, and CD45 in undifferentiated canine MSCs.1
Fig. 1 Flow cytometric expression of CD73, CD90, CD105, and CD45 in undifferentiated canine MSCs.1
Reference
For Research Use Only. Not For Clinical Use.