Glycosylation Analysis Service for HIV Glycoprotein
HIV Glycoprotein
Human immunodeficiency virus (HIV) belongs to the Retroviridae virus family and is primarily responsible for the development of acquired immune deficiency syndrome (AIDS). The viral genomic RNA of HIV contains nine genes that encode essential components for viral replication within susceptible host cells. One of the critical components of HIV is the envelope glycoprotein (Env), which is composed of two subunits: gp120 and gp41 subunits. Gp120 is the outermost glycoprotein subunit and plays a key role in binding to host cell receptors, specifically CD4 receptors found on the surface of T cells, macrophages, and dendritic cells. Gp41 mediates viral envelope fusion with the host cell membrane, facilitating viral entry. Env is heavily glycosylated and is extensively modified by high-mannose glycans, and complex glycans in some cases. Understanding the glycosylation patterns of the Env glycoprotein is instrumental in comprehending HIV infection and advancing vaccine and drug development efforts.
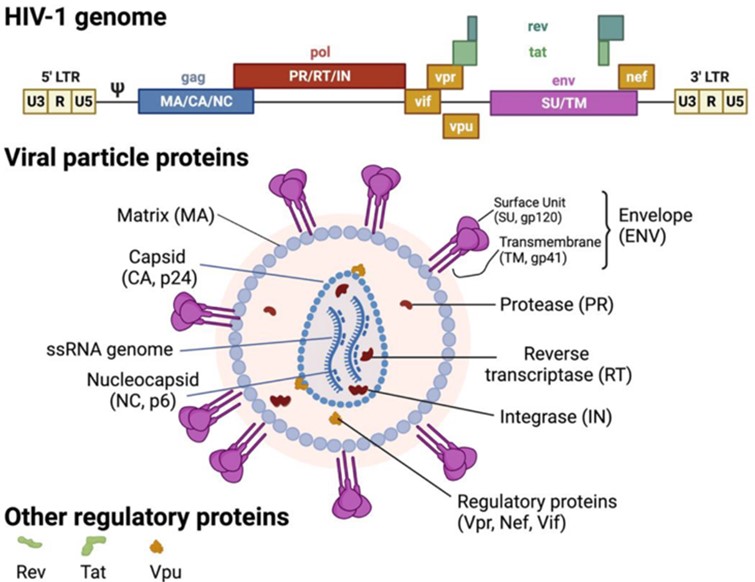 Fig.1 HIV-1 genome and virion structure.1, 3
Fig.1 HIV-1 genome and virion structure.1, 3
HIV Glycosylation Analysis Services at Creative Biolabs
A comprehensive and in-depth analysis of Env glycosylation is essential for informing the design of vaccines and antiviral therapeutics. Creative Biolabs has been at the forefront of this effort, offering robust glycosylation analysis services aimed at uncovering the glycosylation patterns of Env derived from diverse cell types. Our approach involves meticulous site-specific analysis of HIV Env, harnessing a repertoire of advanced technologies, including sample preparation, selective enzymatic cleavage, various chromatographic methods (such as HPLC and HILIC), and cutting-edge MALDI-TOF mass spectrometry. Each of these techniques is strategically deployed to target distinct aspects of glycan or glycoprotein structure. Through our comprehensive analysis, we acquire a multifaceted understanding of the glycosylation patterns on HIV Env.
Application of Env Glycosylation Analysis
Developing an HIV-1 vaccine is a paramount public health priority to combat the HIV-1 pandemic. HIV Env is currently the primary target for eliciting broadly neutralizing antibodies (bNAbs) against the virus. Glycosylation analysis of HIV Env at Creative Biolabs may facilitate the development of vaccines through several key points:
-
Glycan protection
The dense glycosylation of Env acts as a shield, protecting the virus from immune recognition and neutralization.
-
Binding epitopes
Glycans on Env are integral to the binding sites recognized by bNAbs. Mapping these glycan-dependent epitopes is critical for vaccine design.
-
Sialic acid content
Decreasing the sialic acid content of glycans may enhance the immune system's response to Env.
-
High-mannose glycans
High-mannose glycans are essential for the binding of some bNAbs. Changes in glycosylation can affect the binding to antibodies.
In addition to vaccine design, glycosylation of HIV Env is also a primary target of antiviral treatments. Several noteworthy developments include the discovery of BanLec, an isolated lectin exhibiting potent anti-HIV-1 activity, and the isolation of bNAbs against HIV-1 with specific recognition of glycan-dependent epitopes. Moreover, synthetic glycomimetic ligands have shown success in competitively inhibiting HIV-1 binding and infection of immune cells.
Highlights

Precise mapping
We leverage advanced mass spectrometry detection methods and the latest high-performance equipment to achieve precise mapping and unravel the site-specific glycosylation features of heavily glycosylated Env proteins.

Systematic profiling
We utilize both intact glycopeptides and deglycosylated peptides to systematically profile the N-glycoaylation sites, N-glycan site occupancy, and N-glycan types at each glycosylation site, and determination of sialic acid content.

In-depth characterization
Utilizing different ionization modes, fragmentation techniques, and database searching tools, our focus on intact glycopeptides significantly enhances the identification of glycopeptides and provides accurate determinations of site-specific occupancy by diverse glycoforms.

Revealing new insights
In our profiling of intact N/O-glycopeptides, we possess the capability to unveil previously undiscovered glycosylation sites and decipher the unique glycans associated with each site.
Published Data
Technology: MS-based site-specific N-glycosylation analysis
Journal: Nature Communications
IF: 17.692
Published: 2017
Results: The Env trimer is protected by N-linked glycans, concealing the protein from immune detection. Evaluating the glycan type at each glycosylation site can aid in designing improved vaccines. The author developed a method to assess the occupancy and ratio of high-mannose to complex-type glycans at all HIV-1 Env glycosylation sites. This method involves using various proteases for enhanced sequence coverage and multiple peptides per glycosylation site.
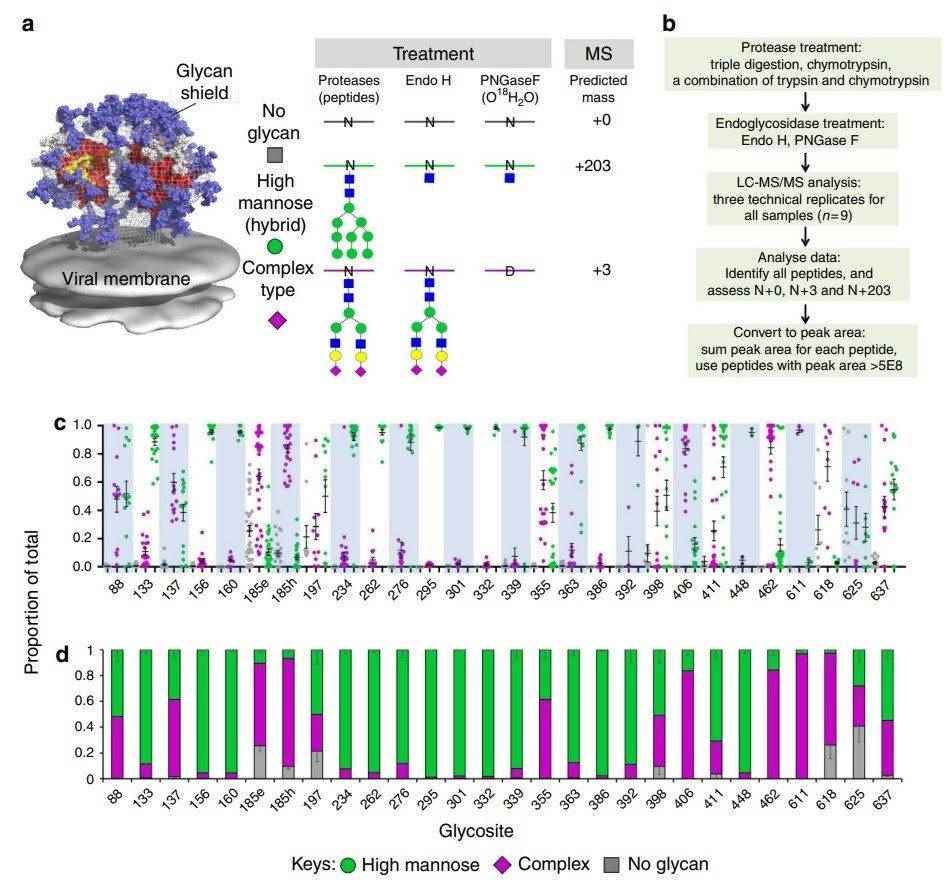 Fig.2 Site-specific glycosylation analysis of HIV-1 Env.2, 3
Fig.2 Site-specific glycosylation analysis of HIV-1 Env.2, 3
Studying Env glycosylation is a critical component of HIV research and is pivotal in the ongoing efforts to develop effective vaccines and therapeutics against HIV/AIDS. Creative Biolabs remains dedicated to offering comprehensive glycosylation services. Our commitment to cutting-edge techniques and thorough analysis ensures that we deliver valuable insights into this complex field. If you need more information or have specific inquiries, please don't hesitate to contact us.
References
-
Proulx, Jessica, et al. "HIV-1-Mediated acceleration of oncovirus-related non-AIDS-defining cancers." Biomedicines 10.4 (2022): 768.
-
Cao, Liwei, et al. "Global site-specific N-glycosylation analysis of HIV envelope glycoprotein." Nature Communications 8.1 (2017): 14954.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 HIV-1 genome and virion structure.1, 3
Fig.1 HIV-1 genome and virion structure.1, 3




 Fig.2 Site-specific glycosylation analysis of HIV-1 Env.2, 3
Fig.2 Site-specific glycosylation analysis of HIV-1 Env.2, 3