Glycosylation Analysis Service for COVID-19 Glycoprotein
COVID-19 Glycoprotein
The COVID-19 pandemic, stemming from SARS-CoV-2 infection, ranks as one of human history's most widespread and devastating pandemics. A key player in this viral interaction is the spike (S) protein of SARS-CoV-2, classified as a class I viral fusion protein. This protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor on the cell surface, facilitating membrane fusion and the virus's entry into host cells. The S protein bears significant glycosylation, featuring 22 predicted N-glycosylation consensus sites and numerous mucin-type O-glycosylation sites. Notably, this S protein stands out as a primary target in the development of vaccines and therapeutics against COVID-19. Glycosylation of the S protein serves vital roles, including proper folding and the introduction of structural diversity, impacting the interaction between the virus and its receptor. Additionally, it yields a glycan shield that complicates antibody neutralization. A comprehensive understanding of the structure and function of the S protein and its glycosylation is paramount for developing vaccines and therapeutics against SARS-CoV-2.
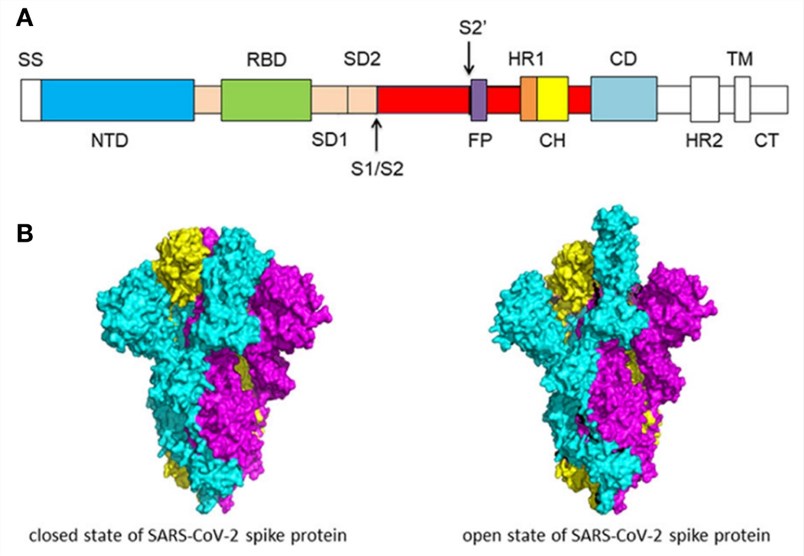 Fig.1 Schematic of SARS-CoV-2 spike protein structure1, 3
Fig.1 Schematic of SARS-CoV-2 spike protein structure1, 3
Glycosylation Analysis Services for SARS-CoV-2 Glycoprotein at Creative Biolabs
Creative Biolabs offers a comprehensive suite of glycosylation analysis services, supported by versatile glycoprotein analysis platforms. Our expertise enables us to conduct compositional and structural profiling of the SARS-CoV-2 S glycoprotein. We extend our glycosylation analysis services to cover both recombinant S protein purified from various expression systems and native S protein isolated from SARS-CoV-2 infected cell lines. It has been reported that distinct expression systems yield S proteins with divergent glycosylation patterns encompassing glycan composition and type variations. We perform site-specific MS/MS-based glycosylation analysis, incorporating electron-transfer/higher-energy collision dissociation (EThcD) techniques. This approach allows us to generate an extensive glycosylation map of the S protein, encompassing well-characterized compositional and structural profiling, along with site occupancy assessment for both N- and O-glycosylation patterns.
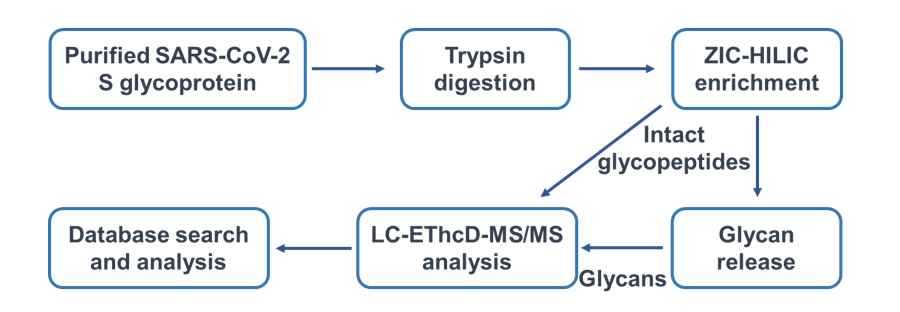 Fig.2 Workflow of site-specific glycosylation analysis of SARS-CoV-2 S glycoprotein. (Creative Biolabs Original)
Fig.2 Workflow of site-specific glycosylation analysis of SARS-CoV-2 S glycoprotein. (Creative Biolabs Original)
Applications of Cell Glycoengineering for Minimizing Glycan Heterogeneity
The presence of heterogeneity in the N-glycan structures of therapeutic proteins introduces difficulties in protein purification, product consistency, and batch-to-batch reproducibility. Furthermore, excessive heterogeneity can pose a safety risk due to the potential for non-human glycans to trigger undesirable immune responses. Manipulating heterogeneity to achieve uniform glycan structures is crucial for maintaining the potency and safety of glycoprotein biologics, as well as regulating their pharmacokinetic properties and improving therapeutic efficacy.
Sample Types Available
-
Purified recombinant SARS-CoV-2 S glycoprotein
-
Cell lines expressing recombinant SARS-CoV-2 spike protein
Glycosylation Analysis Services
-
Glycopeptide mapping
-
Site occupancy analysis
-
Glycan characterization
-
Glycan antennary profiling
-
Glycan heterogeneity analysis
-
Comparison analysis of different strains
Highlights
-
Site-specific analysis at intact glycopeptides level
-
High-resolution and accuracy of MS/MS detection
-
Specialized SOP for glycan analysis
-
Professional team experienced in glycosylation analysis
-
High service quality with a short turnaround time
Applications

Vaccine development

Neutralizing antibody development
Manufacture of diagnostic cassettes

Pathological studies on SARS-CoV-2
Published Data
Technology: MS-based glycan analysis
Journal: Science
IF: 63.832
Published: 2020
Results: The authors conducted a comprehensive analysis of the N-linked glycan structure and site occupancy on the recombinant SARS-CoV-2 S protein purified from HEK293F cells, employing a site-specific mass spectrometric method. In this study, all 22 glycans attached to the SARS-CoV-2 S protein were identified, and their respective abundances were categorized into oligomannose, hybrid, and various forms of complex-type glycosylation based on branching and fucosylation patterns.
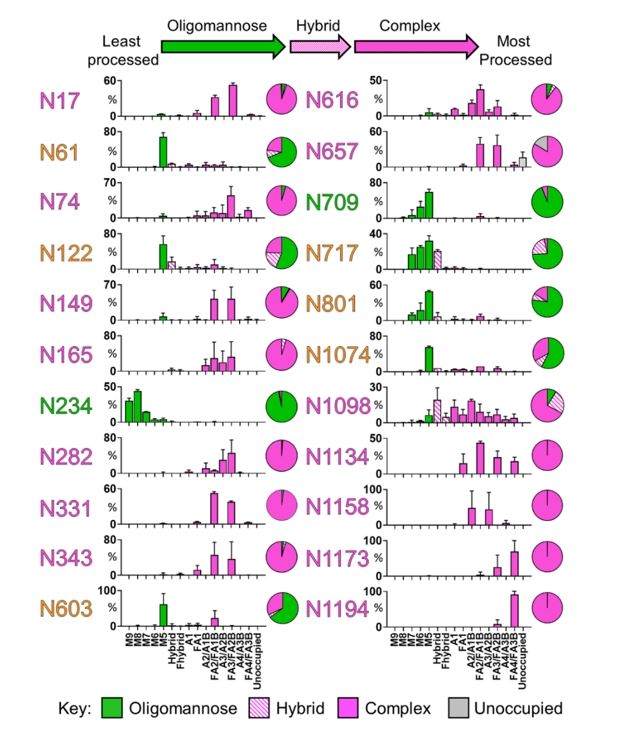 Fig.3 Site-specific N-linked glycosylation of SARS-CoV-2 S glycoprotein.2, 3
Fig.3 Site-specific N-linked glycosylation of SARS-CoV-2 S glycoprotein.2, 3
Creative Biolabs is dedicated to providing invaluable insights into the glycosylation of the SARS-CoV-2 glycoprotein, contributing to a deeper understanding of its structure and function in the pursuit of effective solutions against COVID-19. Please feel free to contact us for more information or send an inquiry directly.
References
-
Wang, Mei-Yue, et al. "SARS-CoV-2: structure, biology, and structure-based therapeutics development." Frontiers in Cellular and Infection Microbiology 10 (2020): 587269.
-
Watanabe, Yasunori, et al. "Site-specific glycan analysis of the SARS-CoV-2 spike." Science 369.6501 (2020): 330-333.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Schematic of SARS-CoV-2 spike protein structure1, 3
Fig.1 Schematic of SARS-CoV-2 spike protein structure1, 3
 Fig.2 Workflow of site-specific glycosylation analysis of SARS-CoV-2 S glycoprotein. (Creative Biolabs Original)
Fig.2 Workflow of site-specific glycosylation analysis of SARS-CoV-2 S glycoprotein. (Creative Biolabs Original)




 Fig.3 Site-specific N-linked glycosylation of SARS-CoV-2 S glycoprotein.2, 3
Fig.3 Site-specific N-linked glycosylation of SARS-CoV-2 S glycoprotein.2, 3

