Glycosylation Analysis Service for Dengue Virus Glycoprotein
Dengue Virus Glycoproteins
Dengue Virus (DENV) is an enveloped, positive-sense, single-stranded RNA virus belonging to the Flaviviridae family. The DENV genome is approximately 10.6 kb and is translated into a polyprotein which includes three structural proteins: capsid (C), envelope (E), and (pre)membrane protein (prM/M), as well as seven non-structural (NS) proteins, known as NS1-5. N-glycosylation sites of DENV proteins have been identified in E, NS1, and prM/M. The E protein plays a crucial role during viral entry by interacting with host carbohydrate receptors through N-glycosylation sites and attached carbohydrate chains, it is also a primary target for neutralizing antibodies. NS1, secreted during DENV infection, induces various host responses and contributes to immune evasion. N-glycosylation on the E and NS1 proteins plays a vital role in the DENV infection processes. Both E and NS1 are key targets for developing vaccine candidates.
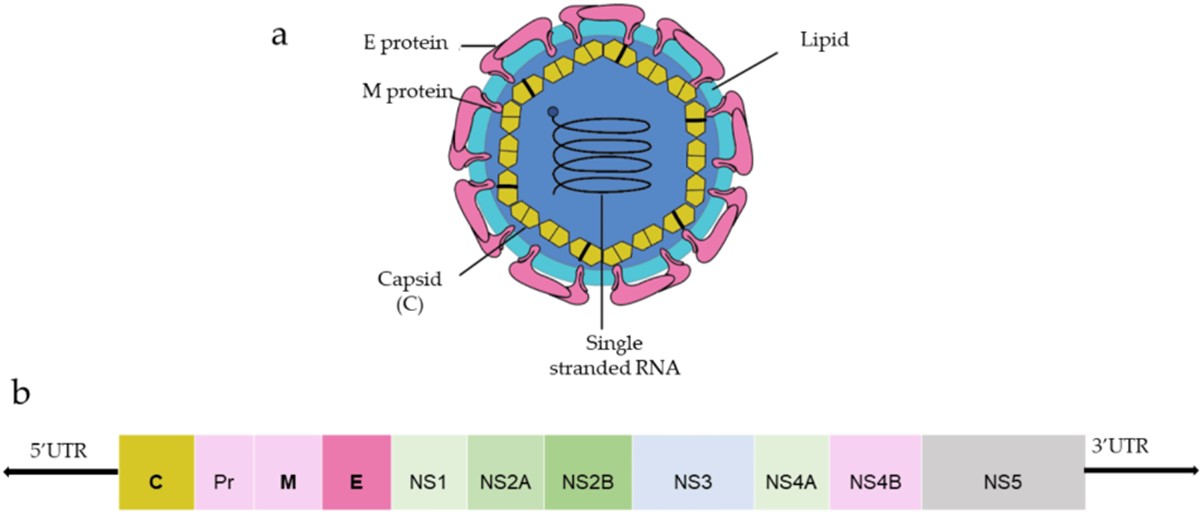 Fig.1 General structure and genome organization of DENV.1, 2
Fig.1 General structure and genome organization of DENV.1, 2
Glycosylation Analysis Services for DENV Glycoprotein at Creative Biolabs
To develop effective therapies against DENV, it is crucial to conduct further research to comprehensively understand the complex glycosylation of DENV and its interaction with carbohydrate receptors. Creative Biolabs has established versatile glycosylation analysis platforms, including lectin microarray and site-specific N-glycosylation analysis with LC-ESI-MS/MS or LC-MALDI-MS/MS, to unravel the glycosylation patterns and decipher the infection mechanism. These strategies provide detailed information on N-glycans attached to DENV glycoproteins across different serotypes, including DENV1, 2, 3, and 4. These analyses profile the structure and sugar composition of N-glycans at each glycosylation site and allow for a comparative analysis of glycan heterogeneity, providing insights into how DENV interacts with carbohydrate receptors.
Glycosylation Analysis of Different DENV Glycoproteins
-
E glycoprotein: The E protein features two N-linked glycosylation sites, N67 and N153. N67 is reported to interact directly with host cell receptors like DC-SIGN, while N153 represents a conserved glycosylation site. These glycosylation patterns vary among different serotypes.
-
NS1 glycoprotein: The NS1 protein contains two N-linked glycosylation sites, N130 and N207, each with distinct types of N-glycans. The N130-glycans are complex glycans, while the N207-glycans consist of high mannose-type glycans.
-
prM/M glycoprotein: The prM protein, containing a glycosylation site at N69, plays a role in the fusion of the E protein. Additional potential N-linked glycosylation sites have also been reported.
Our in-depth glycosylation analysis of these glycoproteins, each with distinct roles in the DENV infection cycle, provides a comprehensive understanding of the mechanisms underlying DENV infection, which is instrumental in advancing the development of diverse antiviral drugs and vaccines.
Applications of Glycosylation Analysis of DENV Glycoproteins
-
Development of vaccine candidates targeted to DENV E and NS1 protein
-
Development of therapeutics targeting viral glycosylation processes
-
Development of DENV entry inhibitors targeting virus–carbohydrate receptor interactions
Creative Biolabs, with extensive experience in glycoproteomics, offers comprehensive viral glycosylation analysis services to elucidate the glycosylation patterns of various DENV glycoproteins. Our services provide valuable insights into viral infection mechanisms and contribute to the development of vaccines. If you have specific requirements or inquiries related to viral glycoprotein analysis, please feel free to contact us for detailed information and assistance.
References
-
Taslem Mourosi, Jarin, et al. "Nucleic acid vaccine platform for dengue and zika flaviviruses." Vaccines 10.6 (2022): 834.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 General structure and genome organization of DENV.1, 2
Fig.1 General structure and genome organization of DENV.1, 2

