Therapeutic Glycoprotein Production in Insect Cells
Many protein drugs are glycosylated and the attached glycan structures often influence the therapeutic properties. The number and composition of the glycans play an important role in protein folding, solubility, and intracellular trafficking . Glycosylation is a very critical modification of therapeutic proteins, known to significantly modulate yield, bioactivity, solubility, stability against proteolysis, immunogenicity, and clearance rate from circulation. Insect cells are able to produce recombinant proteins with complex glycans, but with structures quite different from the human. Currently, the only therapeutics approved for insect cell expression systems are the human papillomavirus vaccine, the prostate cancer immunotherapy vaccine, and the flu vaccine.
Insect Expression Systems
Basic research yielded two insect cell-based recombinant protein production systems known as the baculovirus-insect cell system (BICS) and the Drosophila expression system. Each is now widely used as a tool to produce recombinant proteins and glycoproteins for basic and industrial biomedical research. The insect cell-baculovirus expression system is a powerful platform to rapidly produce a high level of r-proteins. The main insect cell lines used are Spodoptera frugiperda SF9 or SF21 and Trichoplusia ni BTI 5B1-4. Owing to the high-mannose and paucimannose type of glycosylation that is obtained in insect cells, no therapeutic protein is currently produced in this system as this would compromise in vivo bioactivity and potentially induce allergenic reactions. Engineering those cells with glycosyltransferases allows the production of proteins with mammalian-type sugars. Insect cells also offer an interesting platform to produce vaccine antigens or virus-like particles, while engineered baculoviruses containing mammalian promoters (bacmam) show great potential as genetic vectors for mammalian cells transduction. Glycoengineering approaches to produce complex, human-like N-glycans are being developed to enable the human therapeutic use of recombinant glycoprotein production by the BEV-insect cells platform.
Glycosylation Properties of Insect Cells Production Platforms for Biotherapeutic Proteins
These represent an alternative system for the efficient expression of processed recombinant proteins. However, recombinant proteins derived from insect cells also carry glycan structures that differ significantly from those present in mature human glycoproteins, including paucimannose type glycans where 3-7 mannose residues are predominant. Insect cell N-glycans may also contain immunogenic α1-3fucose attached to the innermost GlcNAc residue, in contrast to mammals, which exclusively synthesize core α 1-6 fucose residues. Although insect cells have the ability to perform most of the mammalian-like posttranslational modifications, this system is unable to perform sialylation, which is essential for serum half-life and biological activity of the protein.
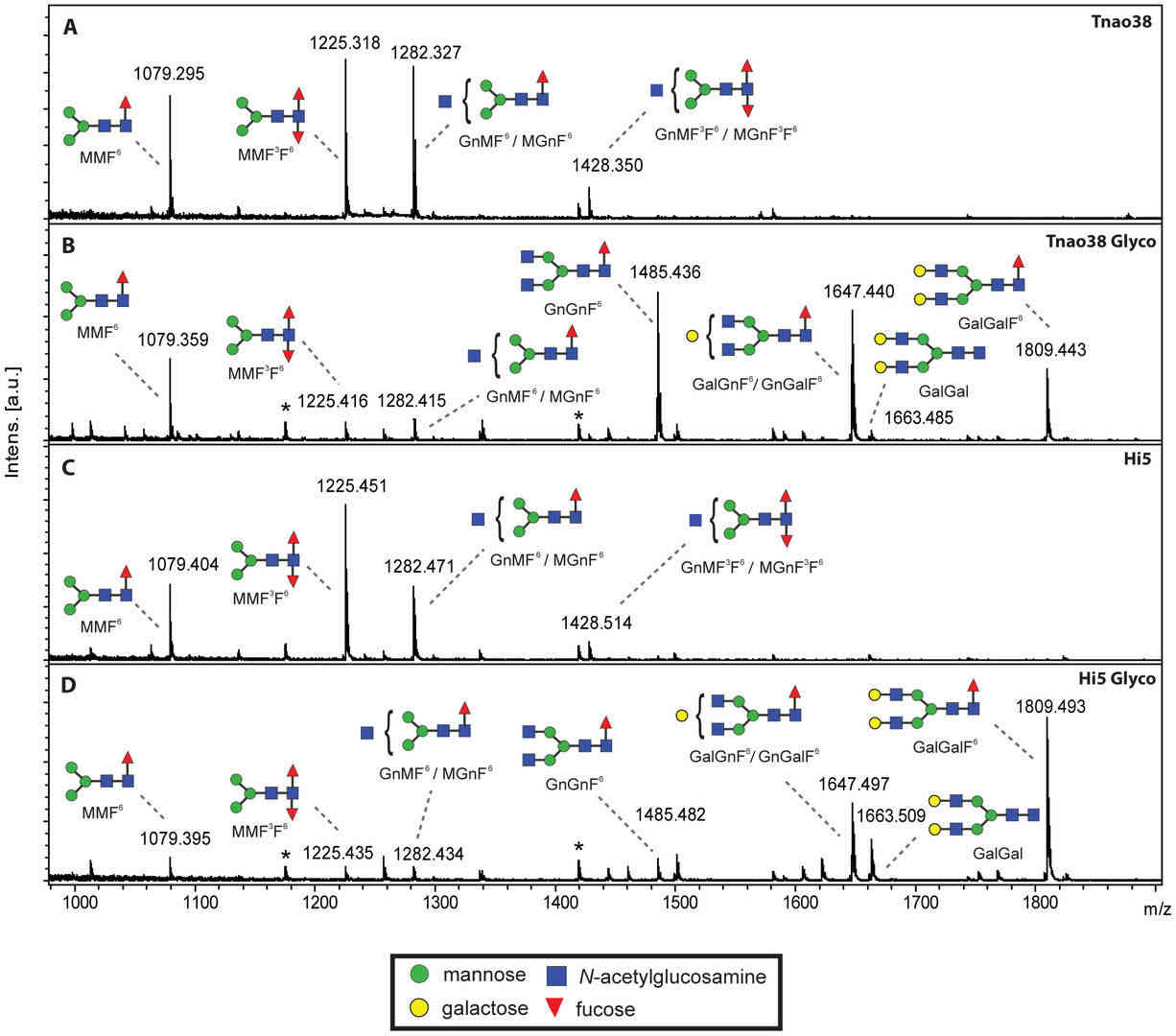 Fig.1 Analysis of glycoprotein glycoforms produced by insect expression cells.1
Fig.1 Analysis of glycoprotein glycoforms produced by insect expression cells.1
Insect protein glycosylation pathways are complex and tissue- and/or developmental stage-specific. Insect cells have the protein N-glycosylation machinery needed to produce and transport lipid-linked oligosaccharides, transfer them to newly synthesized polypeptides, and trim the resulting N-linked oligosaccharide precursors to produce hybrid-type trimannosyl core structures. In addition, insect cells express a specific, processing N-acetylglucosaminidase known as fused lobes (FDL), which is not found in mammalian cells and removes the terminal N-acetylglucosamine residue from the lower (α3) branch of hybrid-type trimannosyl core structures. Thus, the most highly processed N-glycans produced by insect cell lines used in the BICS and DES have paucimannose structures. Insects also have mucin-type protein O-glycosylation machinery.
Glyco-Engineering Insect Expression Systems
Generally, one can identify the specific glycoengineering steps required to improve the glycoprotein processing capabilities of insect-based expression systems by identifying the relevant functions that are missing, relative to mammalian cells. Once identified, introducing these functions could eliminate the differences between insect and mammalian protein glycosylation pathways. Creative Biolabs has built a comprehensive glyco-engineering platform that offers multiple expression systems and therapeutic glycoprotein development services.
Creative Biolabs combines innovative R&D, a keen knowledge of the latest trends, and state-of-the-art research to develop proven glycoprotein solutions that add a unique competitive edge to your product. We pride ourselves on being an innovator and problem solvers in the glycoprotein industry. If you are interested in any of our services or technologies, please feel free to contact us for more.
Reference
-
Palmberger, Dieter, et al. "SweetBac: a new approach for the production of mammalianised glycoproteins in insect cells." PLoS One 7.4 (2012): e34226. Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Analysis of glycoprotein glycoforms produced by insect expression cells.1
Fig.1 Analysis of glycoprotein glycoforms produced by insect expression cells.1

