Therapeutic Glycoprotein Production in Yeast
The yeast-based expression system is considered very attractive and has been extensively used for the production of relevant proteins. Recent advances in the expression of therapeutic glycoproteins with humanized glycan structures in engineered yeast have made significant progress. Almost 20% of the biopharmaceuticals are being produced by S. cerevisiae. Another yeast-based system is Pichia pastoris, considered a successful heterologous expression system with several recombinant proteins in clinical trials.
Yeast Expression Systems
The yeast-based expression systems have been used for the production of several approved therapeutic proteins including recombinant insulin. Protein production in yeast offers several advantages, such as robust expression, scalable fermentation, and the ability to perform N-glycosylation. The availability of an expression system that has the ability to customize the glycosylation of a therapeutic protein is likely to lead to the development of improved therapeutic glycoproteins. The use of glycoengineered yeast to control glycosylation could generate tissue-targeted therapeutic glycoproteins such as interferon, where targeting to the liver might overcome dose-limiting systemic toxicities.
Engineering N-Glycosylation in Yeast
Not only is N-glycosylation important in the folding process of most recombinant proteins, but it also has a large impact on the pharmacokinetics and pharmacodynamics of the therapeutic proteins. Glycosylation of the protein can increase its hydrodynamic volume, reducing renal clearance. Next to the impact on clearance, glycosylation of the protein may offer protection against proteolytic degradation. Glyco-engineering of yeast cells includes the removal of yeast-specific glycosylation, sometimes followed by the construction of hybrid- or complex-type (human) glycans. Next to the modification of the type of N-glycan structures on the protein, considerable effort has been devoted to reducing the macro- and microheterogeneity present on glycoproteins. A first step in the humanization of N-glycosylation in yeast is the removal of the high-mannose and hypermannosyl structures. Two main strategies are followed, one based on the elimination of yeast glycosyltransferases and the other one on interference in the assembly of the LLO. A subsequent step is the introduction of different glycosyltransferases and glycosidases to obtain hybrid- and complex-type N-glycans. A recent, third approach, which efficiently converts yeast N-glycosylation into a type that is often function-neutral, is the expression of an endo-β-N-acetylglucosaminidase, capable of removing high-mannose N-glycans and resulting in a largely deglycosylated product.
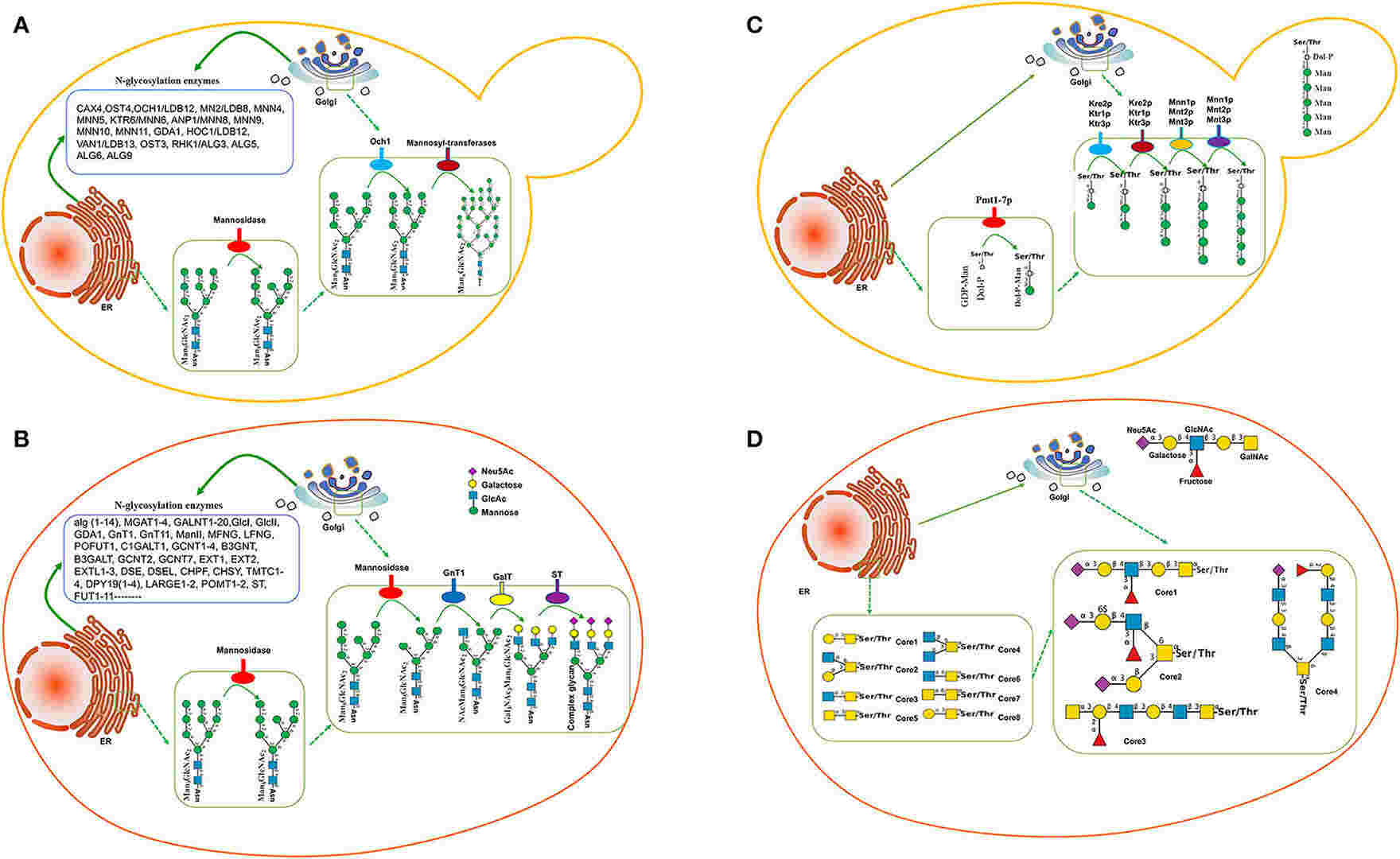 Fig.1 Comparison of glycosylation pathways in yeast and mammalian cells.1, 2
Fig.1 Comparison of glycosylation pathways in yeast and mammalian cells.1, 2
Examples of Recombinant Proteins Expressed in Glyco-Engineered Yeast Strains
-
Production of Monoclonal Antibodies
Monoclonal antibodies (mAbs) constitute a large portion of biopharmaceuticals on the market. Their production relies heavily on posttranslational modification. P. pastoris-produced anti-HER2 shows increased ADCC activity, probably due to the lack of core fucose residues. Next to an enhanced ADCC, P. pastoris-produced mAbs show a more homogeneous glycosylation profile compared to the large heterogeneity of complex-type N-glycans in CHO cells.
-
Enzyme Replacement Therapies
Lysosomal storage diseases are orphan diseases that, in some cases, can be treated with enzyme replacement therapy (ERT). For the treatment of Pompe disease, the ERT, acid glucosidase α (GAA), is mainly targeted to the muscle cells via the cation independent mannose-6-phosphate receptor (CI-MPR). To increase the mannose-6-phosphate to substantial levels, the Mnn4p ortholog of S. cerevisiae was overexpressed in P. pastoris, which resulted in glycoproteins carrying N-glycans, of which 80% contained at least one mannose-6-phosphate.
Some of the most important vaccines are produced in yeasts, being vaccines against the human hepatitis B virus (HBV) and the human papillomavirus (HPV).
With the advent of humanized yeast strains and their ability to exercise direct control over glycosylation, we see the advantages of mammalian cell culture fading and expect a significant number of biopharmaceuticals to be switched to yeast-based expression platforms. At Creative Biolabs, we challenge ourselves to think out of the box and innovate. The trust of our customers is of paramount importance to us. Our team of experts is engrossed in delivering the best quality you deserve. You can count on our glyco-engineered Pichia pastoris expression system for your needs, we promise to deliver. With our experience and expertise at your service, please contact us for more.
Reference
-
Li, Xingjuan, et al. "Humanization of yeasts for glycan-type end-products." Frontiers in Microbiology 13 (2022): 930658. Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Comparison of glycosylation pathways in yeast and mammalian cells.1, 2
Fig.1 Comparison of glycosylation pathways in yeast and mammalian cells.1, 2

