Therapeutic Glycoprotein Production in Mammalian Cells
The number of recombinant proteins produced by mammalian expression systems is increasing as post-translational modifications of biological agents are of increasing concern, especially their glycosylated status. Appropriate glycosylation can improve the properties of recombinant proteins, such as increasing their stability and half-life in blood circulation and reducing their immunogenicity. CHO (Chinese hamster ovary) cell line is the most widely used mammalian cell line.
Cell Hosts
CHO Cells
CHO cells are widely used for glycoprotein production because of their numerous advantages. These cells can achieve a substantial production rate, are suitable for large-scale industrial suspension culture, and can be adapted to grow in various serum-free and chemically defined culture media. The generated products from CHO cells are to more likely be compatible and bioactive within human hosts. Furthermore, these cells are refractory to infection by human viruses, which minimizes biosafety risks for commercial production purposes. Moreover, different gene amplification systems have been developed and used in CHO cells, which allow for high titer yields and good specific productivity. There are many examples of biotherapeutic glycoproteins approved by the Food and Drug Agency (FDA) and the European Medicines Agency (EMA) currently produced in these cells. Although CHO cells possess many advantages for glycoprotein productions, they are unable to produce some types of human glycosylation, such as α-2,6-sialylation and α-1,3/4-fucosylation.
Human Cell Lines
One way to favor human-like glycosylation would be to use human cell lines for recombinant protein production. The most commonly used human cell lines to manufacture glycoprotein therapeutics are the HEK293 cells and the HT-1080, respectively from human embryo kidney and fibrosarcoma origin. Some human cell lines are currently being used in preclinical and/or clinical development stages for recombinant glycoprotein production. CL184 is a combination of two mAbs used against the rabies virus. The HKB-11 cell line is a fusion of HEK293S and human B-cell lines. It has recently shown high-level protein production and α 2,3 and α 2,6-sialic acid linkages. Two other cell lines, the CAP cells of human amniocytes origin and the HuH-7 cells of human hepatocellular carcinoma origin, are presently tested for recombinant glycoprotein production in preclinical phases, and both display human-like glycosylation profiles.
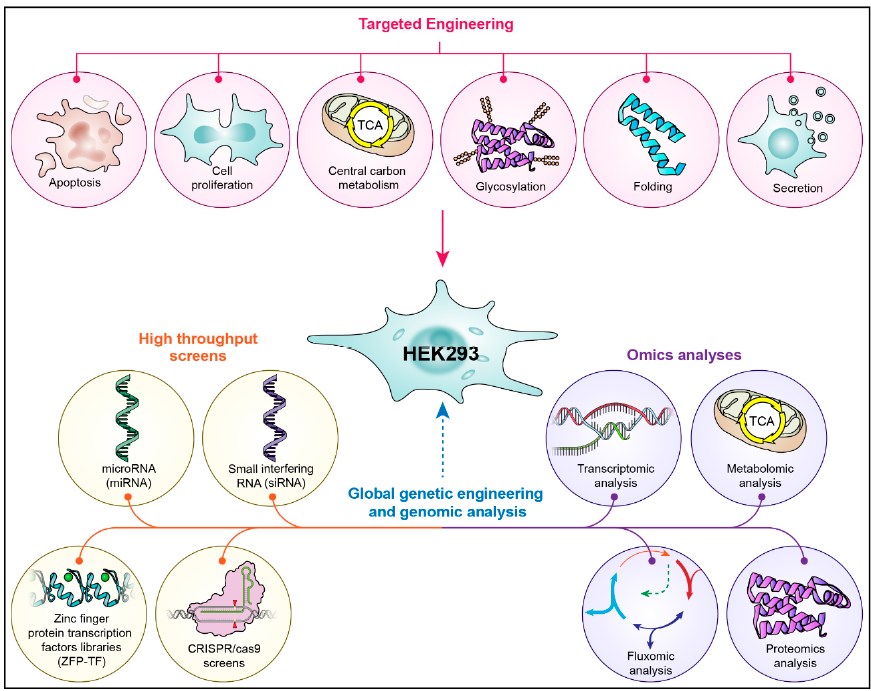 Fig.1 Engineering the HEK293 cell line for improved culture performance.1, 2
Fig.1 Engineering the HEK293 cell line for improved culture performance.1, 2
Other Mammalian (non-human) Cell Lines
Baby Hamster Kidney (BHK) cells are mostly been used for the production of vaccines. Only two marketed recombinant glycoproteins are currently manufactured in these cells. Murine myeloma cells (NS0 and Sp2/0), derived from tumor cells also are being used to produce some commercial monoclonal antibodies. Murine cells can also produce α-gal and Neu5Gc at considerably higher levels than hamster cells, increasing the risks of immunogenicity.
Glycoengineering
The expression systems used for glycoprotein productions have significantly different glycosylation machinery. To avoid any potential immunogenic response, glycans present on biotherapeutic proteins should be compatible with human hosts. Consequently, several glycoengineering strategies have emerged to recreate these beneficial profiles on recombinant proteins. In the biopharmaceutical industry, the production of glycoproteins is currently achieved by either transient or stable gene expression in the cells. When the need for a quick and economical approach prevails, the transient expression remains the best choice for protein production. When it comes to the production of glycoproteins in large quantities, stable gene expression systems remain the preferred avenue. For these systems, multiple aspects have been tackled and optimized for improving productivity, process robustness, and reducing cell line generation timelines. You can choose our mature Glyco-engineered Mammalian Cell Expression System as the best choice for your project.
Mammalian cell lines, in particular CHO cells, are now extensively used for the production of therapeutic glycoproteins by the biopharmaceutical industry. These cells possess many advantages in terms of cell culture and have the capacity to generate high titers. Creative Biolabs has been focusing on glycoprotein research for many years and has established a mature mammalian cell expression system. Our experts and the technical team all have professional discipline backgrounds. We pride ourselves on being an innovator and problem solvers in glycoprotein research. If you are interested in our services and technologies, please feel free to contact us for more details.
Reference
-
Abaandou, Laura, David Quan, and Joseph Shiloach. "Affecting HEK293 cell growth and production performance by modifying the expression of specific genes." Cells 10.7 (2021): 1667. Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Engineering the HEK293 cell line for improved culture performance.1, 2
Fig.1 Engineering the HEK293 cell line for improved culture performance.1, 2

