Therapeutic Glycoprotein Production in Plant Cells
In recent years, the pharmaceutical industry has put considerable effort into the development of novel glycoprotein therapeutics. The use of genome editing tools, the extensive integration of analytical data, and a better understanding of cellular processes will facilitate the development of next-generation expression hosts for custom-made expression of recombinant glycoprotein pharmaceuticals. These glycoengineering approaches will allow easier production of biosimilar drugs and lead to the generation of bio-better pharmaceuticals with altered glycosylation and optimized efficacy and safety.
Plant-based Systems for Therapeutic Glycoprotein Production
Genetically modified plants are especially well suited for the production of edible oral vaccines. Since they offer significant potential in reducing the cost of manufacturing glycosylated and pathogen-free therapeutics, many plant-based expression systems are under development. Plant cells can synthesize complex protein and also glycoproteins, showing greater similarity to humans in terms of N-glycan structure. Accordingly, there is a recent and growing interest in using plant cells to produce biopharmaceuticals by biotechnology companies around the world. Elelyso, intended for the treatment of Gaucher’s disease, was the first recombinant glycoprotein produced by plant cells.
N-glycan Biosynthesis is Similar in Plants and Mammals
In plant cells, as in other eukaryotic cells, protein N-glycosylation starts in the ER by the co- or post-translational transfer of an oligosaccharide precursor, Glc3Man9GlcNAc2, from a dolichol lipid carrier onto specific Asn residues constitutive of the N-glycosylation consensus sequence Asn-X-Ser⁄Thr. Once transferred onto the nascent protein and while the glycoprotein is transported along the secretory pathway, eventually, the addition of new sugar residues in the ER and the GA to build up complex-type N-glycans. Whereas high-Man-type N-glycans contain five to nine Man residues and have the same structure in plant and mammalian glycoproteins, plant complex-type N-glycans are structurally different from their mammalian counterparts.
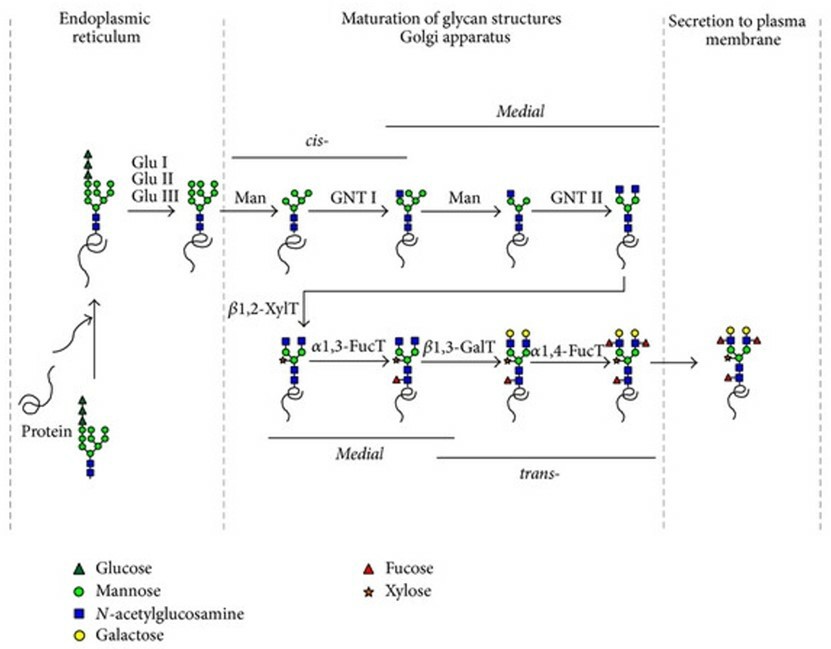 Fig.1 N-glycosylation pathways in plants.1
Fig.1 N-glycosylation pathways in plants.1
N-glycosylation Engineering in Plant Expression Systems
Considerable progress has been made in recent years to redesign protein N-glycan structures in plant systems, using different strategies.
Targeted Expression to the ER
Proteins resident of the ER lumen contains high-Man-type N-glycans structurally similar in plant and mammalian cells. Taking advantage of the conserved C-terminal signals H/KDEL for protein retention in the ER, KDEL tags were fused to both the heavy and light chains of recombinant antibodies for targeted expression in the ER. PMPs with high-Man-type N-glycans also offer many other potentials, but yet unexploited applications, involving notably their targeting to the Man-specific cell surface receptors of macrophages and dendritic cells.
Knockout Strategies to Prevent the Addition of Unwanted Sugar Residues
Gene inactivation or silencing may be used to reduce or eliminate the activity of plant-specific glycosyltransferases. The availability of protein N-glycosylation mutants in Arabidopsis has also allowed for the production of therapeutic glycoproteins devoid of plant-specific glycoepitopes.
Knockin of Heterologous Glycosyltransferases for the Production of Galactosylated and Sialylated N-glycans
Most circulating human glycoproteins are N-glycosylated with oligosaccharides that are “capped” by neuraminic acid (NeuAc) on penultimate β1,4 galactose residues. The absence of terminal sialic acid residues on glycosylated PMPs reduces their clinical efficiency because of a rapid clearance via the galactose-specific hepatic asialoglycoprotein receptor. This positive impact of sialic acid on protein stability likely explains why knockin strategies for glycoengineering in plants have mainly focused on the addition of terminal β1,4 galactose and sialic acid residues to humanize N-glycans. The targeting of glycosyltransferases to specific subcellular locations could strongly improve the efficiency of knockin strategies for protein glycoengineering. Supporting this, recent papers have illustrated the potential of expressing a mammalian glycosyltransferase precisely targeted along with the plant cell secretory pathway by fusing its catalytic domain to the localization domain of a plant glycosyltransferase.
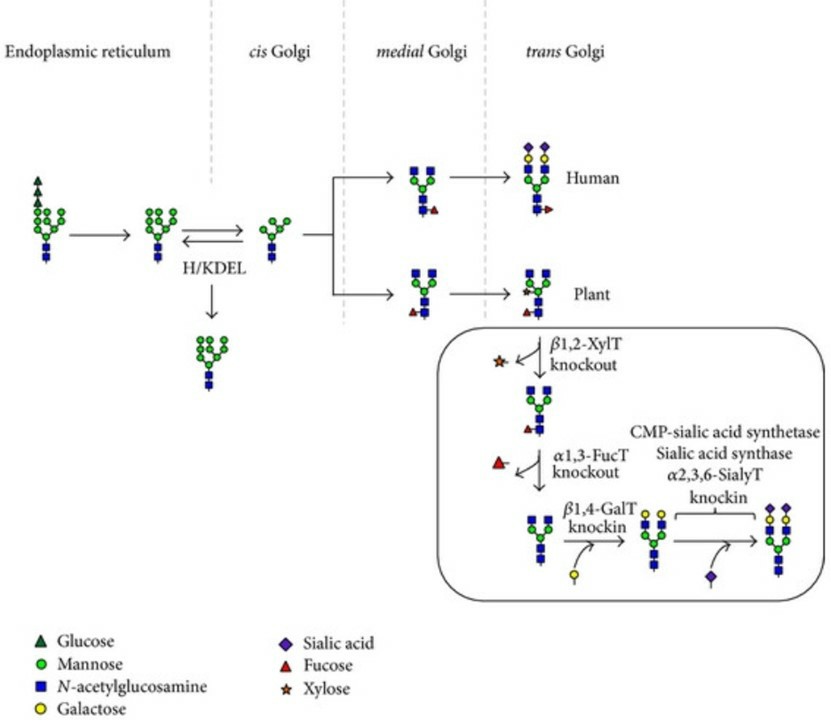 Fig.2 Schematic diagram of humanization of the glycosylation pathway in plant.1
Fig.2 Schematic diagram of humanization of the glycosylation pathway in plant.1
The development of plant platforms dedicated to the production of glycosylated PMPs for parenteral application in humans requires the consideration of both N- and O-glycan-specific glycoepitopes. Engineering N-glycosylation in plants could improve the efficiency of PMPs, not only by reducing plant N-glycan immunogenicity in humans but also by providing plant expression systems dedicated to the production of therapeutic protein glycovariants hardly produced at low cost in cultured mammalian cells. Creative Biolabs delivers a high-quality glyco-engineered plant-based expression system, award-winning project design, and convenient fulfillment services that save customers’ time, money, and stress. If you are interested in our services or technologies, please feel free to contact us for more.
Reference
-
Kim, Hyun-Soon, et al. "N‐Glycosylation Modification of Plant‐Derived Virus‐Like Particles: An Application in Vaccines." BioMed research international 2014.1 (2014): 249519. Distributed under Open Access license CC BY 3.0, without modification.
For Research Use Only.
Resources

 Fig.1 N-glycosylation pathways in plants.1
Fig.1 N-glycosylation pathways in plants.1
 Fig.2 Schematic diagram of humanization of the glycosylation pathway in plant.1
Fig.2 Schematic diagram of humanization of the glycosylation pathway in plant.1

