Therapeutic Glycoprotein Production in Bacteria
About 70% of human therapeutic proteins are N-linked glycoproteins, so host cells used for production must contain the relevant protein modification mechanisms. The discovery and identification of N-linked glycosylation pathways in pathogenic bacteria Campylobacter jejuni and subsequently transfer its function to E. coli provide an opportunity to use prokaryotes as cell factories for the production of therapeutic proteins.
Engineering of Mammalian N-Glycan Structures in E. coli
The expression of bacterial oligosaccharyltransferase invests E. coli cells with the ability to N-glycosylate proteins and would therefore allow the expression of therapeutically interesting N-glycoproteins in this host. Bacterial N-glycan structures differ greatly from mammalian ones and are therefore often immunogenic. To produce glycoproteins carrying mammalian N-glycan structures as therapeutics in bacteria, extensive alteration of the bacterial glycan structures is necessary. Approaches taken to solve this problem include genetic engineering of the glycosylation pathway and enzymatic remodeling of glycan structures. A more direct way to humanize bacterial N-glycans is therefore the bottom-up assembly of synthetic glycosylation pathways to produce glycan structures that more closely resemble mammalian ones.
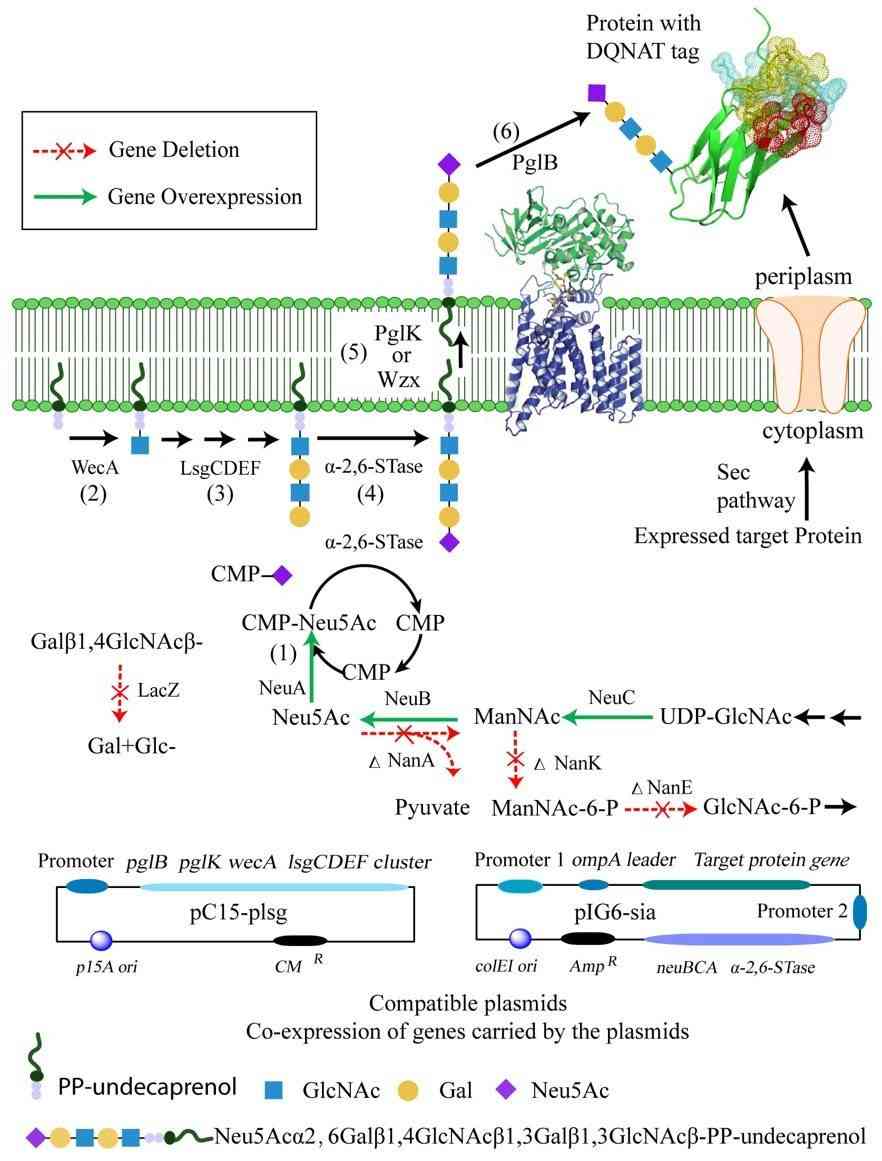 Fig.1 Schematic diagram of E. coli glycoengineering.1, 3
Fig.1 Schematic diagram of E. coli glycoengineering.1, 3
Glycosylation in Bacteria
It is useful here to examine the advantages of using a bacterium such as E. coli to produce human glycoproteins. From a molecular standpoint, E. coli has a published genome sequence and is facile to genetically modify. Generally, bacteria can produce higher titers of the product even compared to yeast and are certainly cheaper to culture with shorter fermentation times. More specifically, bacteria are less sensitive to glycosylation changes, and essential cell survival is not dependent upon the glycosylation process, and cell death is therefore unlikely. This means glycosylation control is more amenable in E. coli, leading to less heterogeneous glycoprotein products. Further, controlling glycan type and site can lead to increased efficacy. The potential to modify protein structure, half-life, immunogenicity, cellular uptake, and target recognition is extremely exciting, and bacteria would be an ideal host compared to eukaryotes where hundreds of enzymes are involved, complicating process control. In bacteria, the N-glycosylation process occurs independently from protein translocation machinery, and therefore fully folded proteins can be glycosylated.
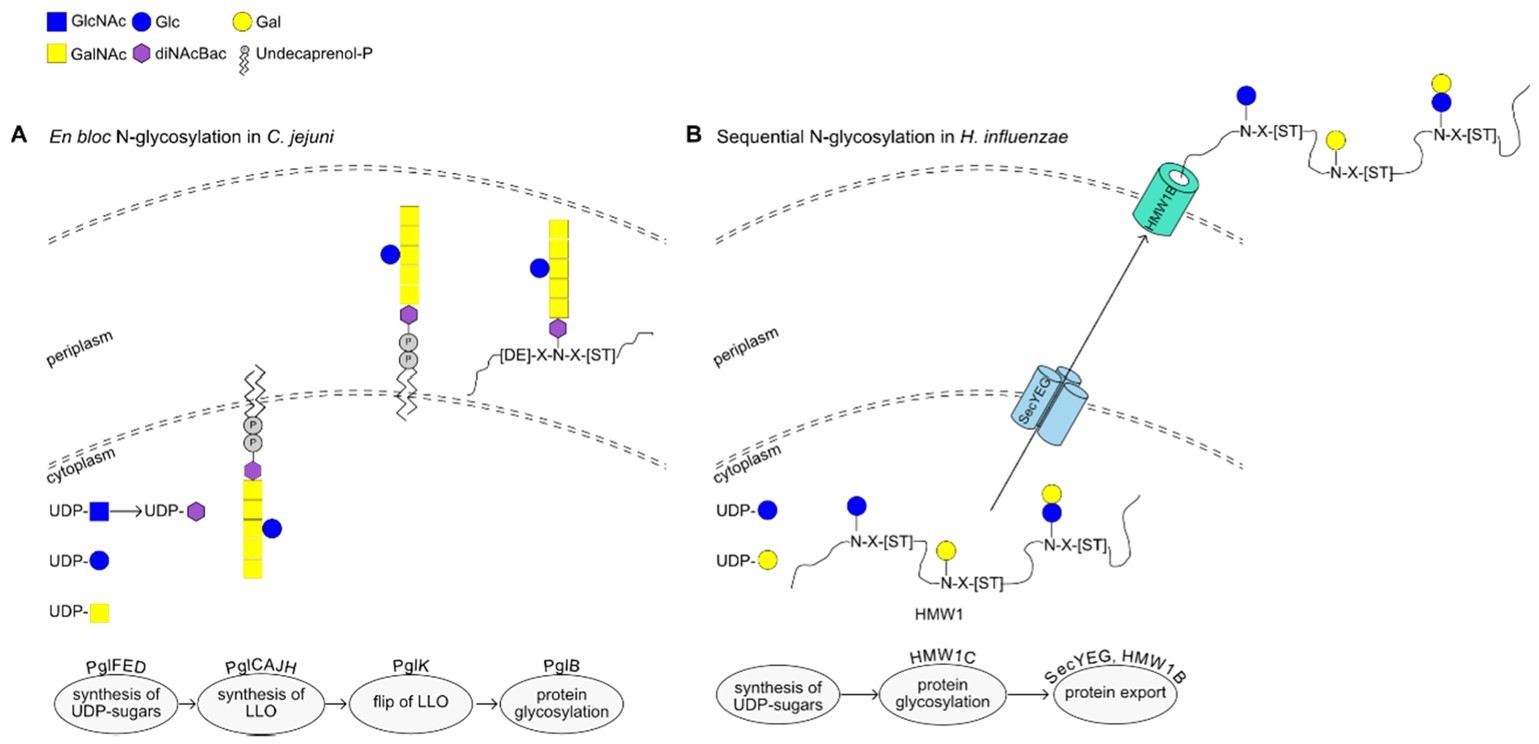 Fig.2 Examples of N-glycosylation in bacteria.2, 3
Fig.2 Examples of N-glycosylation in bacteria.2, 3
Bacterial glycoengineering has experienced a rise in popularity following the discovery of bacterial N-glycosylation systems with the ability to generate non-native glycans and conjugate these to recombinantly expressed proteins. Glycoengineering in E. coli for human therapeutic drug production holds a potentially sweet future. The use of E. coli as an expression host for producing human therapeutic glycoproteins is most likely suited for smaller and less complex proteins than produced in CHO cells.
When you choose Creative Biolabs as your service provider, a planning expert will guide you through the project development process. This value-added service helps expedite the preparation of your work order and greatly benefits the project outcome. We're ready to help you with therapeutic glycoprotein development and production, please contact us to get started.
References
-
Zhu, Jing, et al. "An engineered pathway for production of terminally sialylated N-glycoproteins in the periplasm of Escherichia coli." Frontiers in Bioengineering and Biotechnology 8 (2020): 313.
-
Latousakis, Dimitrios, and Nathalie Juge. "How sweet are our gut beneficial bacteria? A focus on protein glycosylation in Lactobacillus." International journal of molecular sciences 19.1 (2018): 136.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Schematic diagram of E. coli glycoengineering.1, 3
Fig.1 Schematic diagram of E. coli glycoengineering.1, 3
 Fig.2 Examples of N-glycosylation in bacteria.2, 3
Fig.2 Examples of N-glycosylation in bacteria.2, 3

