Stromal interaction molecule 1 (STIM1) is a type I transmembrane protein located mainly in the endoplasmic reticulum (ER), with a significant pool of approximately 20% at the plasma membrane. It is encoded by the STIM1 gene which is located in the region of chromosome 11p15.5. STIM1 consists of an N-terminal EF-hand domain that binds calcium in the ER lumen, a sterile alpha motif (SAM) that mediates STIM oligomerization, and a C-terminal cytosolic region (Fig.1). The cytoplasmic C terminus comprises two coiled-coil (CC), a serine/proline rich (S/P) and a polybasic lysine-rich (K) domain and regulates CRAC channel activation. STIM1 is a Ca²⁺ sensor that partners with Orai1 to constitutively binds Ca²⁺ in the ER luminal space to sense its store depletion. A decrease in ER Ca²⁺ results in loss of binding of STIM1 to Ca²⁺, which leads to the clustering of this protein into multimeric complexes that then activate Orai1 in the plasma membrane to generate a Ca²⁺-selective channel that reloads the ER directly from the extracellular space.
| Basic Information of STIM1 | |
| Protein Name | Stromal interaction molecule 1 |
| Gene Name | STIM1 |
| Organism | Homo sapiens (Human) |
| UniProt ID | Q13586 |
| Transmembrane Times | 1 |
| Length (aa) | 685 |
| Sequence | MDVCVRLALWLLWGLLLHQGQSLSHSHSEKATGTSSGANSEESTAAEFCRIDKPLCHSEDEKLSFEAVRNIHKLMDDDANGDVDVEESDEFLREDLNYHDPTVKHSTFHGEDKLISVEDLWKAWKSSEVYNWTVDEVVQWLITYVELPQYEETFRKLQLSGHAMPRLAVTNTTMTGTVLKMTDRSHRQKLQLKALDTVLFGPPLLTRHNHLKDFMLVVSIVIGVGGCWFAYIQNRYSKEHMKKMMKDLEGLHRAEQSLHDLQERLHKAQEEHRTVEVEKVHLEKKLRDEINLAKQEAQRLKELREGTENERSRQKYAEEELEQVREALRKAEKELESHSSWYAPEALQKWLQLTHEVEVQYYNIKKQNAEKQLLVAKEGAEKIKKKRNTLFGTFHVAHSSSLDDVDHKILTAKQALSEVTAALRERLHRWQQIEILCGFQIVNNPGIHSLVAALNIDPSWMGSTRPNPAHFIMTDDVDDMDEEIVSPLSMQSPSLQSSVRQRLTEPQHGLGSQRDLTHSDSESSLHMSDRQRVAPKPPQMSRAADEALNAMTSNGSHRLIEGVHPGSLVEKLPDSPALAKKALLALNHGLDKAHSLMELSPSAPPGGSPHLDSSRSHSPSSPDPDTPSPVGDSRALQASRNTRIPHLAGKKAVAEEDNGSIGEETDSSPGRKKFPLKIFKKPLKK |
Due to its ability to act as an ER-intraluminal Ca²⁺ sensor, STIM1 regulates store-operated Ca²⁺ entry (SOCE), which is the major extracellular Ca²⁺ influx pathway involved in a wide variety of signaling pathways in excitable and, particularly, in non-excitable cells. After depletion of Ca²⁺ in ER, STIM1 relocates to the region adjacent to the plasma membrane in the ER, then promotes the opening of Orai1 in the plasma membrane and induces extracellular Ca²⁺ entry, and activates T cell factor through nuclear factor of activated T cells (NFAT). In addition, STIM1 also interacts with the transient receptor potential channels (TRPC) in various cell types. STIM1 knockdown inhibits up-regulation of TRPC1 and leads to the abolishment of SOCE and a strong decrease in NFAT activation, which results in the inhibition of cardiomyocyte hypertrophy. Moreover, STIM1 is originally identified as an anti-metastatic gene in melanoma cells, and it is later found that STIM1 overexpression significantly enhances local spread and angiogenesis, and promotes cancer cell migration and invasion. Knockout of STIM1 may result in a reduction in cancer metastasis, in hepatocellular carcinoma, melanoma and colorectal cancer.
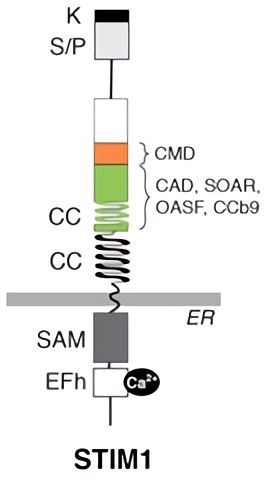 Fig.1 STIM1 protein domains. STIM1 contains an N-terminal EF-hand domain and a sterile alpha motif (SAM), and a cytoplasmic C terminus domain that containing two coiled-coil (CC), a serine/protein rich (S/P) and a polybasic lysine-rich (K) domain. A minimal CRAC channel binding and activation domain (alternatively termed CAD, SOAR, OASF, CCb9) and an adjacent CRAC modulatory domain (CMD) mediating fast inactivation of CRAC currents are shown.
Fig.1 STIM1 protein domains. STIM1 contains an N-terminal EF-hand domain and a sterile alpha motif (SAM), and a cytoplasmic C terminus domain that containing two coiled-coil (CC), a serine/protein rich (S/P) and a polybasic lysine-rich (K) domain. A minimal CRAC channel binding and activation domain (alternatively termed CAD, SOAR, OASF, CCb9) and an adjacent CRAC modulatory domain (CMD) mediating fast inactivation of CRAC currents are shown.
This article finds that STIM1 plays an important role in paraquat-induced cell cycle arrest and cell death in acute lung injury, which may provide new insights to target paraquat-induced intoxication.
This article suggests that STIM1 deletion protects the liver from ischemia-reperfusion (I/R) injury in mice by inhibiting inflammation, oxidative stress and apoptosis, and it can be used as a potential therapeutic target for improving liver I/R injury.
This study shows that STIM1 haplo-insufficient mice exhibit a maladaptive response to cardiac stress overload.
This study uses breast cancer tissue for immunohistochemical analysis to assess STIM1 expression and finds that STIM1 expression is present in 66.1% of breast cancer cases, which is significantly higher than adjacent non-tumor tissues.
This article suggests that STIM1 knockdown can inhibit migration and invasion of A549 cells in vitro, as well as metastasis in vivo.
Membrane protein research has made significant progress in recent years. Based on our versatile Magic™ membrane protein production platform, we offer a range of protein preparation services to our customers worldwide in a variety of active forms of membrane proteins. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-STIM1 antibody development services.
During the past years, Creative Biolabs has successfully produced a number of functional membrane proteins for customers worldwide. If you would like to ask for more details, please feel free to contact us.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.