Introduction of ABCC9
ABCC9, also known as SUR2, ATFB12, ABC37, CANTU, CMD1O, or ATP-binding cassette, subfamily C (CFTR/MRP), member 9, codes for a polypeptide of 1549 amino acids which is named as sulfonylurea receptor 2 (SUR2). It is a 174.2 kDa membrane protein and in humans located on the chromosome 12p12.1. The protein produces a subunit of a channel that transports potassium ions across cell membranes. Each of the channels is composed of eight subunits, four ABCC9 proteins and four proteins generated from either the KCNJ8 gene or KCNJ11 gene. The subunits of ABCC9 regulate the activity of these channels, deciding whether it is open or closed. Some diseases correlated with ABCC9 are Cantu syndrome and familial atrial fibrillation. And among its associated pathways are inwardly rectifying K+ channels and cardiac conduction.
| Basic Information of ABCC9 | |
| Protein Name | ATP-binding cassette sub-family C member 9 |
| Gene Name | ABCC9 |
| Aliases | Sulfonylurea receptor 2 |
| Organism | Homo sapiens (Human) |
| UniProt ID | O60706 |
| Transmembrane Times | |
| Length (aa) | 1549 |
| Sequence | MSLSFCGNNISSYNINDGVLQNSCFVDALNLVPHVFLLFITFPILFIGWGSQSSKVQIHHNTWLHFPGHNLRWILTFALLFVHVCEIAEGIVSDSRRESRHLHLFMPAVMGFVATTTSIVYYHNIETSNFPKLLLALFLYWVMAFITKTIKLVKYCQSGLDISNLRFCITGMMVILNGLLMAVEINVIRVRRYVFFMNPQKVKPPEDLQDLGVRFLQPFVNLLSKATYWWMNTLIISAHKKPIDLKAIGKLPIAMRAVTNYVCLKDAYEEQKKKVADHPNRTPSIWLAMYRAFGRPILLSSTFRYLADLLGFAGPLCISGIVQRVNETQNGTNNTTGISETLSSKEFLENAYVLAVLLFLALILQRTFLQASYYVTIETGINLRGALLAMIYNKILRLSTSNLSMGEMTLGQINNLVAIETNQLMWFLFLCPNLWAMPVQIIMGVILLYNLLGSSALVGAAVIVLLAPIQYFIATKLAEAQKSTLDYSTERLKKTNEILKGIKLLKLYAWEHIFCKSVEETRMKELSSLKTFALYTSLSIFMNAAIPIAAVLATFVTHAYASGNNLKPAEAFASLSLFHILVTPLFLLSTVVRFAVKAIISVQKLNEFLLSDEIGDDSWRTGESSLPFESCKKHTGVQPKTINRKQPGRYHLDSYEQSTRRLRPAETEDIAIKVTNGYFSWGSGLATLSNIDIRIPTGQLTMIVGQVGCGKSSLLLAILGEMQTLEGKVHWSNVNESEPSFEATRSRNRYSVAYAAQKPWLLNATVEENITFGSPFNKQRYKAVTDACSLQPDIDLLPFGDQTEIGERGINLSGGQRQRICVARALYQNTNIVFLDDPFSALDIHLSDHLMQEGILKFLQDDKRTLVLVTHKLQYLTHADWIIAMKDGSVLREGTLKDIQTKDVELYEHWKTLMNRQDQELEKDMEADQTTLERKTLRRAMYSREAKAQMEDEDEEEEEEEDEDDNMSTVMRLRTKMPWKTCWRYLTSGGFFLLILMIFSKLLKHSVIVAIDYWLATWTSEYSINNTGKADQTYYVAGFSILCGAGIFLCLVTSLTVEWMGLTAAKNLHHNLLNKIILGPIRFFDTTPLGLILNRFSADTNIIDQHIPPTLESLTRSTLLCLSAIGMISYATPVFLVALLPLGVAFYFIQKYFRVASKDLQELDDSTQLPLLCHFSETAEGLTTIRAFRHETRFKQRMLELTDTNNIAYLFLSAANRWLEVRTDYLGACIVLTASIASISGSSNSGLVGLGLLYALTITNYLNWVVRNLADLEVQMGAVKKVNSFLTMESENYEGTMDPSQVPEHWPQEGEIKIHDLCVRYENNLKPVLKHVKAYIKPGQKVGICGRTGSGKSSLSLAFFRMVDIFDGKIVIDGIDISKLPLHTLRSRLSIILQDPILFSGSIRFNLDPECKCTDDRLWEALEIAQLKNMVKSLPGGLDAVVTEGGENFSVGQRQLFCLARAFVRKSSILIMDEATASIDMATENILQKVVMTAFADRTVVTIAHRVSSIMDAGLVLVFSEGILVECDTVPNLLAHKNGLFSTLVMTNK |
Function of ABCC9 Membrane Protein
The ABCC9 protein is a member of the multidrug resistance-associated protein (MRP) subfamily, a subunit of the ATP-binding cassette (ABC) transporter superfamily. This protein is thought to constitute ATP-sensitive potassium (K-ATP) channels in cardiac, skeletal, as well as vascular and non-vascular smooth muscle. ABCC9, together with KCNJ11, forms cardiac and smooth muscle-type K-ATP channels. It is required for the activation and regulation of the channel while KCNJ11 makes up the channel pore. The channels open and close are in response to the amount of ATP (the main energy source of cells) inside the cell. The resulting transport of potassium ions is part of a complex network of signals that transmit chemical messages into and out of cells. Based on the protein structure of ABCC9, some reports suggest a role as the drug-binding channel-modulating subunit of the extra-pancreatic K-ATP channels. In addition, since ABCC9 is observed in most human tissues and relevant to many human diseases, it has been considered as a target for therapeutic strategies.
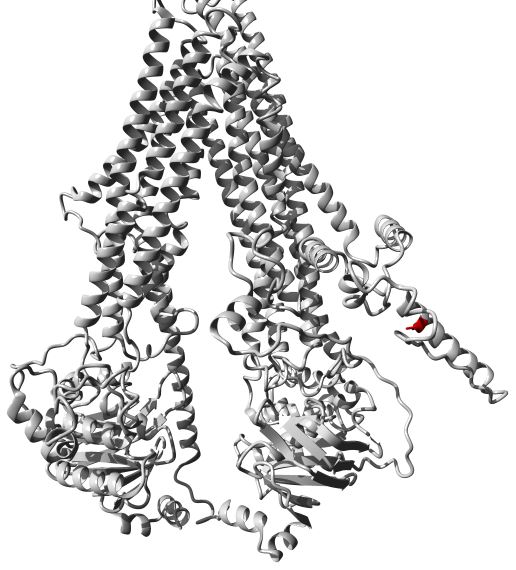 Fig.1 Molecular model of the ABCC9 protein. (Harakalova, 2012)
Fig.1 Molecular model of the ABCC9 protein. (Harakalova, 2012)
Application of ABCC9 Membrane Protein in Literature
This report concluded mutations in CS-related SUR2 resulted in KATP gain-of-function. They contributed to linking CS genotypes to phenotypes and illustrated the underlying molecular mechanisms, such as consequences for inhibitory drug sensitivity and insights that may inform the development of therapeutic methods to manage CS.
To investigate more about the genetic risk of hippocampal sclerosis of aging (HS-Aging) pathology, gene-based associations of GRN, TMEM106B, ABCC9, and KCNMB2 genes were tested. The HS-Aging pathology protective ABCC9 haplotype was related to the reduced ABCC9 expression, illustrating a possible toxic gain of function.
Cantú syndrome, as a rare disease, was featured by congenital hypertrichosis, osteochondrodysplasia, and cardiomegaly, and was recognized as a rare syndrome. This paper reported the first case of Cantú syndrome in Korea and correlated changes in overall development with rehabilitation over some months.
Here, the patient case mentioned in this study provided further evidence that these syndromes were regarded as an expression of ABCC9-associated disorders, ranging from hypertrichosis as well as acromegaloid facies to a severe end of Cantu syndrome.
ABCC9 genetic polymorphism was related to the increased risk for many human diseases. Here, authors described novel ABCC9 variants in the human brain, corresponding to the altered 3'UTR length, which resulted in targeting by miR-30c. Also, they determined that the HS-Aging risk mutation was correlated with variations in ABCC9 transcript level.
ABCC9 Preparation Options
To produce a high-quality membrane protein of interest, we currently present mature reconstitution forms and active formats for these target proteins through our advanced platforms. Our universal Magic™ membrane protein production platform can ensure several flexible options, and from which our customers can always find a suitable and effective method for their projects. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-ABCC9 antibody development services.
Creative Biolabs as a leading custom service provider in the field of protein markets has won great reputation from global users for successful achieved projects. Today, we are pleased to introduce our expert membrane protein preparation services in required formats. Please feel free to contact us for more information.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.