KCNQ2 is encoded by the KCNQ2 gene which is located on 20q13.3 in human. The molecular mass of KCNQ2 is about 95 kDa. It belongs to the potassium channel family, and the members of which are most wildly expressed ion channels. KCNQ2 can be found in the brain of adult and fetal. And it is mainly expressed in areas containing neuronal cell bodies. Besides, KCNQ2 can be phosphorylated and ubiquitinated, and both posttranslational modifications can influence the biological functions of KCNQ2 in vivo.
| Basic Information of KCNQ2 | |
| Protein Name | Potassium voltage-gated channel subfamily KQT member 2 |
| Gene Name | KCNQ2 |
| Aliases | KQT-like 2, Neuroblastoma-specific potassium channel subunit alpha KvLQT2, Voltage-gated potassium channel subunit Kv7.2 |
| Organism | Homo sapiens (Human) |
| UniProt ID | O43526 |
| Transmembrane Times | Multi-pass membrane |
| Length (aa) | 872 |
| Sequence | MVQKSRNGGVYPGPSGEKKLKVGFVGLDPGAPDSTRDGALLIAGSEAPKRGSILSKPRAGGAGAGKPPKRNAFYRKLQNFLYNVLERPRGWAFIYHAYVFLLVFSCLVLSVFSTIKEYEKSSEGALYILEIVTIVVFGVEYFVRIWAAGCCCRYRGWRGRLKFARKPFCVIDIMVLIASIAVLAAGSQGNVFATSALRSLRFLQILRMIRMDRRGGTWKLLGSVVYAHSKELVTAWYIGFLCLILASFLVYLAEKGENDHFDTYADALWWGLITLTTIGYGDKYPQTWNGRLLAATFTLIGVSFFALPAGILGSGFALKVQEQHRQKHFEKRRNPAAGLIQSAWRFYATNLSRTDLHSTWQYYERTVTVPMYSSQTQTYGASRLIPPLNQLELLRNLKSKSGLAFRKDPPPEPSPSKGSPCRGPLCGCCPGRSSQKVSLKDRVFSSPRGVAAKGKGSPQAQTVRRSPSADQSLEDSPSKVPKSWSFGDRSRARQAFRIKGAASRQNSEEASLPGEDIVDDKSCPCEFVTEDLTPGLKVSIRAVCVMRFLVSKRKFKESLRPYDVMDVIEQYSAGHLDMLSRIKSLQSRVDQIVGRGPAITDKDRTKGPAEAELPEDPSMMGRLGKVEKQVLSMEKKLDFLVNIYMQRMGIPPTETEAYFGAKEPEPAPPYHSPEDSREHVDRHGCIVKIVRSSSSTGQKNFSAPPAAPPVQCPPSTSWQPQSHPRQGHGTSPVGDHGSLVRIPPPPAHERSLSAYGGGNRASMEFLRQEDTPGCRPPEGNLRDSDTSISIPSVDHEELERSFSGFSISQSKENLDALNSCYAAVAPCAKVRPYIAEGESDTDSDLCTPCGPPPRSATGEGPFGDVGWAGPRK |
KCNQ2 can cooperate with another member of KQT (TC 1.A.1.15) subfamily to form a potassium channel of which characteristics are basically similar to the native M-current. At the same time, the current formed by KCNQ2 and KCNQ3 can be inhibited by linopirdine and XE991, and activated by anticonvulsant retigabine. So, KCNQ2 plays a very important role in regulating the excitability of the neurons. Beyond that, KCNQ2 participates in many other biological processes, such as chemical synaptic transmission, ankyrin binding and nervous system development. Accordingly, the mutations of KCNQ2 can cause seizures, benign familial neonatal 1 (BFNS1), and the patients with this disease will occur clusters of seizures in the first days of life. Other kinds of mutations in KCNQ2 are associated with epileptic encephalopathy, early infantile 7 (EIEE7) which is an autosomal dominant seizure disorder. Decreasing the KCNQ2 channel activity in GABAergic parvalbumin interneurons will render the SCZ-predisposing 22q11.2 deletion mice be less capable of inhibiting pyramidal neurons upon D2 modulation.
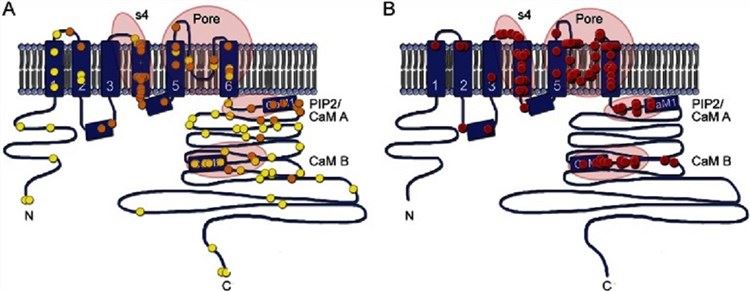 Fig.1 KCNQ2 transmembrane topology. (Millichap, 2016)
Fig.1 KCNQ2 transmembrane topology. (Millichap, 2016)
Accordingly, altered prefrontal cortex may function in schizophrenia (SCZ). Authors in this article find that KCNQ2 channel activity is an important factor in the modulation of prefrontal cortical interneuron activity.
In order to find out the relationship between KCNQ2 encephalopathy genotype and phenotype, authors in this article study 23 patients with the disease. They reveal that the mutations of KCNQ2 can cause neonatal-onset epileptic encephalopathy. And the channel activity of KCNQ2 is crucial in this disease.
Authors in this article report 7 mutations of KCNQ2 and KCNQ3, all of which exhibit a clear loss of function. Children carrying 5 of the 7 KCNQ2 mutations suffer from severe epilepsy and cognitive decline. The phenome may relate to a dominant-negative reduction of the resulting potassium current at subthreshold membrane potentials.
It has been suggested that vitamin B6 has a good effect on seizures in idiopathic epilepsy. Authors in this article report a de novo mutation (c.629G>A; p. Arg210His) in KCNQ2 through Whole-exome sequencing. And then, they reveal some potential mechanisms for the role of vitamin B6 in control seizures.
It has been proved that mutations of KCNQ2 can cause either benign epilepsy or early-onset epileptic encephalopathy (EOEE). Authors in this article study 7 patients with the A294V mutation in KCNQ2. The result suggests that the disease severity is not only contributed by inhibiting M current but also by other mechanisms.
Based on the versatile Magic™ membrane protein production platform, Creative Biolabs can provide many flexible options for soluble and functional target protein, from which you can always find a better match for your particular project. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-KCNQ2 antibody development services.
As a forward-looking biotech company as well as a leading customer service provider in the field of membrane protein, Creative Biolabs has won good reputation among our worldwide customers for successfully accomplishing numerous challenging projects including generation of many functional membrane proteins. Please feel free to contact us for more information.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.