Mouse models play a pivotal role in preclinical studies, allowing for the development and testing of CAR-T cell therapies. A primary source of mouse CAR-T cells serves as an essential resource for exploring the mechanisms that govern CAR-T response and performance post-adoptive transfer in both healthy and disease mouse models. With cutting-edge technologies and extensive expertise in the realm of CAR-T immunotherapy, Creative Biolabs stands ready to supply antigen-specific primary mouse CAR-T cells for evaluating their functionality within the mouse model.
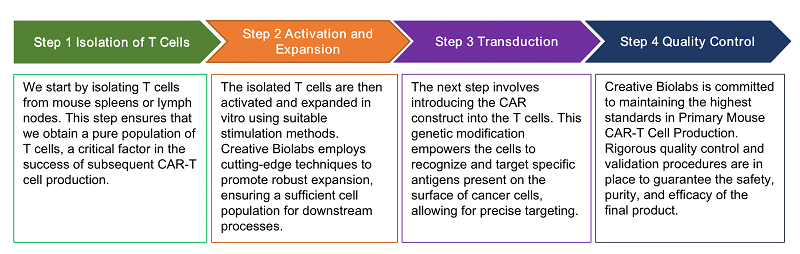
The workflow of our primary mouse CAR-T cell production service is a meticulously designed process that encompasses the following key stages:
Creative Biolabs stands at the forefront of cell therapy, bolstered by a wealth of experience. We provide expert, cutting-edge services to clients worldwide, guiding them towards successful project completion. It is our commitment to advance your projects and expedite progress toward clinical trials through the utilization of our advanced technologies, platforms, and resources.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION