The tumor immune microenvironment (TIME) comprises all immune components of tumors including immune cells, extracellular immune factors and cell surface molecules. The major cells types in TIME are divided into two categories: one is inflammatory and includes active CD8+ T cell, helper1 T cell, dendritic cells (DCs), natural killer (NK) cells, natural killer T cell, M1-like macrophage, and so on; the other one is immunosuppressive and includes M2-like macrophage, regulatory T cell, helper17 T cell, myeloid-derived suppressor cells (MDSCs), inactive CD8+ T cell and so on.
According to lymphocyte infiltration, tumors are classified as 'cold' tumors (showing very low infiltration and not inflamed), 'immune-excluded' tumors (with immune cells aggregating at the tumor boundaries), 'immunosuppressed' (some infiltration but not inflamed) and 'hot' tumors (or 'inflamed' tumors, showing pronounced immune infiltrates in the tumor core). 'Cold' tumors do not elicit a response from the immune system, they do not respond to immunotherapies, while 'Hot' tumors can be infiltrated with T cells working to fight cancer. Therefore heating up the TME to overcome the absence of T cell infiltration allows more effective antitumor immunity.
Immunosuppressive 'cold' tumors are seen to be less responsive to immunotherapy, while immunostimulatory 'hot' tumors are seen to be more responsive to immunotherapy. Turning the immunosuppressive TME of the cold tumor is an efficient strategy in the treatment of cancer. Immunotherapy combinations with conventional chemotherapies, radiation therapies, tyrosine kinase inhibitors and oncolytic viruses are showing promise in turning these 'cold' tumors 'hot'.
With the increasing examination of the TME, it is clear that a number of therapeutic regimens may be successfully repurposed in the treatment of cancer. The refinement of therapeutic targets against immune cells and immunomodulatory molecules within the TME are increasing, and their promise for clinical utility is growing. Modern immunotherapy strategies have been developed based on various approaches, including boosting the anti-tumoral response or relieving immunosuppression.
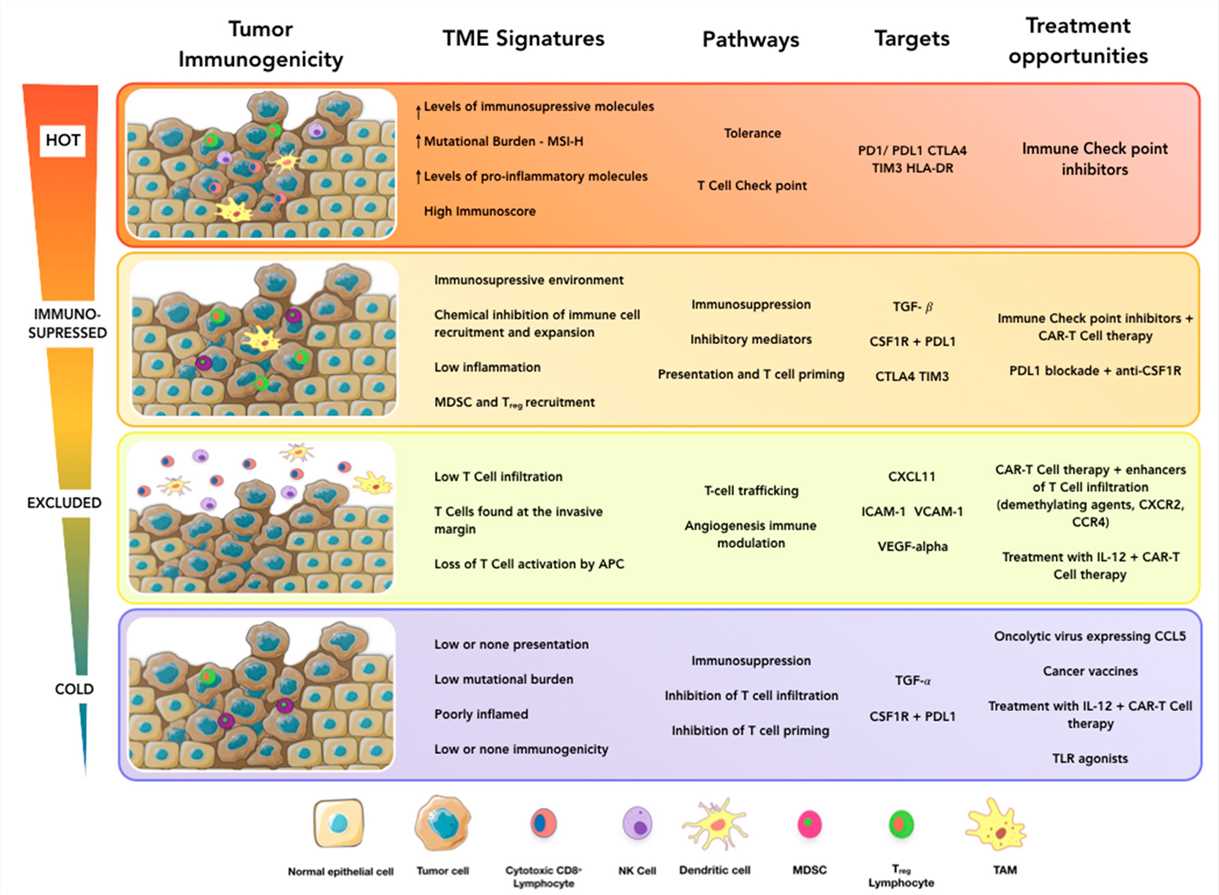 Fig.1 Towards personalized cancer immunotherapy. (Pérez-Romero, 2020)
Fig.1 Towards personalized cancer immunotherapy. (Pérez-Romero, 2020)
The major immune cells involved in cancer progression include tumor-associated macrophages, Teff cells, Treg cells, neutrophils, NK cells and MDSCs. Immune cells interact with the tumor via direct contact or through chemokine and cytokine signaling which shapes the behavior of the tumor and its response to therapy. Many strategies have been developed to target these immune cells. Adoptive cell transfer is one of several immunotherapeutic approaches.
Immune checkpoint blockade (ICB) therapies that enhance the function of anti-tumor T lymphocytes have been especially promising, yielding unprecedented results in the clinic. Depending on the property of checkpoints, this strategy can be achieved by suppressing the inhibitory checkpoints or activating the stimulatory checkpoints with relative antibodies.
Subtle and local changes in intratumoral cytokine profiles form the basis for re-educating or reprogramming the TME. Several cytokines limit tumor cell growth by a direct anti-proliferative or pro-apoptotic activity, or indirectly by stimulating the cytotoxic activity of immune cells against tumor cells or inhibiting the immunosuppressive activity. In particular, interferons (IFN, type I and II), interleukins (IL), and growth factors such as tumor necrosis factor (TNF) have been extensively tested.
Oncolytic viruses (OVs) constitute a new and promising immunotherapeutic approach toward cancer treatment. OVs both directly kill tumor cells and inflame the TME, activating host systemic innate and adaptive immune responses.
With extensive expertise in cancer immunotherapy field, we have developed a range of services to reshape TME, including but not limited to:
In addition, we also provide a series of products to support your research, such as immune cell products, cytokines and chemokine products, etc. If you have any requirements, please feel free to contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION