The tumor microenvironment (TME) consists of cancer cells, stromal tissue, and extracellular matrix. The immune system is an important determinant of the TME. Diverse immune cell types infiltrate the TME, and the dynamic tumor-immune cell interplay gives rise to a rich milieu of cytokines and growth factors. However, tumor surveillance by the immune system may eventually fail.
The infiltrated immune cells are broadly classified into two types: tumor-promoting immune cells and tumor-antagonistic immune cells depending on the composition of immune cells and their phenotypic states. The tumor-antagonizing immune cells mainly consist of effector T cells (including CD8+ cytotoxic T cells and effector CD4+ T cells), natural killer (NK) cells, dendritic cells (DCs), M1-polarized macrophages, and N1-polarized neutrophils. Notably, some infiltrated immune cells serve as tumor-associated immune cells, such as immature/tolerogenic dendritic cells (DCs), M2 macrophages, regulatory T (Treg) cells, and, myeloid-derived suppressor cells (MDSCs). However, numerous findings indicate that almost all types of immune cells present a self-contradictory function under different conditions, although the mechanisms are still unknown.
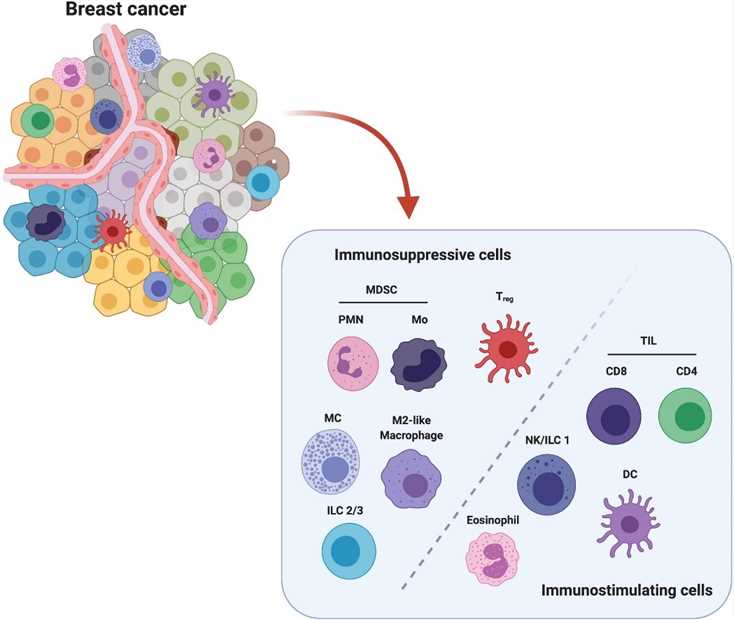 Fig.1 Major players in immune breast TME. (Salemme, 2021)
Fig.1 Major players in immune breast TME. (Salemme, 2021)
The immune cells and the cellular factors produced from them, including both immunosuppressive and inflammatory cytokines, play dual roles in promoting or discouraging cancer development which relies heavily on the TME. At the early stage of carcinogenesis, the immune system eliminates tumor cells via cytotoxic T cell and NK cell killing mechanisms. This is achieved with the help of antigen-presenting cells (APCs). However, with the progressive accumulation of tumor cell mutations and modifications to the TME, the tumor cells can eventually 'escape' from immune surveillance. Multiple lineages of tumor-associated immune cells and various immune mediators such as tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), C-X-C Motif Chemokine Ligand 1 (CXCL-1), CXCL-5, vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMPs), are responsible for shaping a favorable TME for tumor growth. Up-regulation of immune checkpoints is an important aspect of this process. Anti-tumor effector cells, such as CD8+ T cells and NK cells, are suppressed directly or indirectly by TME.
It has been proved that the TME is responsible for tumor immune escape and the ultimate treatment failure. Re-educating the TME to improve the response to cancer immunotherapies can be performed by targeting many cellular and immunosuppressive factors. Targeting immune cells can transform the TME to be pro-inflammatory and relieve immunosuppression. Immunotherapies for tumor suppressor-related immune cells, as well as tumor-promoting immune cells, are being developed one after another. Strategies for targeting immune cells in TME include
As a leading biotechnology company, Creative Biolabs is dedicated to being your trusted solution provider. We have developed a range of services targeting T/NK/ macrophages/MDSCs/DCs cells to reshape TME. Our scientists are always ready to provide the guidance you need to develop cutting-edge immune-modulating therapies. Please feel free to contact us with your requirements.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION