Introduction of LRRC8A
LRRC8A (also known as SWELL1) is a member of the LRRC8 protein family, which is found exclusively in vertebrates and encoded by the LRRC8A gene. LRRC8A is recently shown to be an essential component of volume-regulated anion channel (VRAC) since knockdown or deletion of SWELL1 can abolish ICl swell. LRRC8A is conserved across vertebrate species, and four additional homologous family members (LRRC8B-E) are usually present in their genomes. Deletion and rescue studies have shown that functional VRAC requires LRRC8A and at least one other LRRC8 subunit in heterologous systems, which has revealed subunit interactions by co-immunoprecipitation.
| Basic Information of LRRC8A | |
| Protein Name | Volume-regulated anion channel subunit LRRC8A |
| Gene Name | LRRC8A |
| Aliases | Leucine-rich repeat-containing protein 8A, Swelling protein 1 |
| Organism | Homo sapiens (Human) |
| UniProt ID | Q8IWT6 |
| Transmembrane Times | 4 |
| Length (aa) | 810 |
| Sequence | MIPVTELRYFADTQPAYRILKPWWDVFTDYISIVMLMIAVFGGTLQVTQDKMICLPCKWVTKDSCNDSFRGWAAPGPEPTYPNSTILPTPDTGPTGIKYDLDRHQYNYVDAVCYENRLHWFAKYFPYLVLLHTLIFLACSNFWFKFPRTSSKLEHFVSILLKCFDSPWTTRALSETVVEESDPKPAFSKMNGSMDKKSSTVSEDVEATVPMLQRTKSRIEQGIVDRSETGVLDKKEGEQAKALFEKVKKFRTHVEEGDIVYRLYMRQTIIKVIKFILIICYTVYYVHNIKFDVDCTVDIESLTGYRTYRCAHPLATLFKILASFYISLVIFYGLICMYTLWWMLRRSLKKYSFESIREESSYSDIPDVKNDFAFMLHLIDQYDPLYSKRFAVFLSEVSENKLRQLNLNNEWTLDKLRQRLTKNAQDKLELHLFMLSGIPDTVFDLVELEVLKLELIPDVTIPPSIAQLTGLKELWLYHTAAKIEAPALAFLRENLRALHIKFTDIKEIPLWIYSLKTLEELHLTGNLSAENNRYIVIDGLRELKRLKVLRLKSNLSKLPQVVTDVGVHLQKLSINNEGTKLIVLNSLKKMANLTELELIRCDLERIPHSIFSLHNLQEIDLKDNNLKTIEEIISFQHLHRLTCLKLWYNHIAYIPIQIGNLTNLERLYLNRNKIEKIPTQLFYCRKLRYLDLSHNNLTFLPADIGLLQNLQNLAITANRIETLPPELFQCRKLRALHLGNNVLQSLPSRVGELTNLTQIELRGNRLECLPVELGECPLLKRSGLVVEEDLFNTLPPEVKERLWRADKEQA |
Function of LRRC8A Membrane Protein
The LRRC8 protein forms a heteromeric channel that is important both in structure and function, while VRAC is formed with LRRC8A and other LRRC8 paralogs. VRACs are heteromers consisted of up to five different LRRC8 proteins, with LRRC8A being the only essential subunit. The LRRC8 protein has important structural/functional significance. Specifically, the formation of VRAC requires a protein region of LRRC8A and a protein region of other LRRC8 paralogs. Deletion of LRRC8A abolishes VRAC’s transport of halide anions and a plethora of organic compounds. Truncated LRRC8A mutants are largely stuck in the ER, where they are unable to carry the other LRRC8 subunits (B-E) to the plasma membrane. The truncation of LRRC8A in ébouriffé mice thus results in drastically reduced, but not completely abolished, swelling-activated ICl, vol currents.
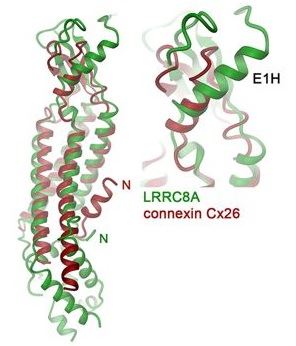 Fig.1 Superposition of subunits of LRRC8A (green) and connexin Cx26 (red, left) and expanded view of the ESD (right). Selected secondary structure elements are indicated. (Deneka, 2018)
Fig.1 Superposition of subunits of LRRC8A (green) and connexin Cx26 (red, left) and expanded view of the ESD (right). Selected secondary structure elements are indicated. (Deneka, 2018)
Application of LRRC8A Membrane Protein in Literature
This article finds that LRRC8A is an essential subunit of VRAC and a key factor for astroglial volume homeostasis.
This paper demonstrates that LRRC8C, LRRC8D or LRRC8E, LRRC8A IL and EL1 are critical for the formation and function of VRAC and provides new insights into channel structure and regulation.
This article reveals the previously unknown architecture of volume-regulated anion channels and their mechanism of selective anion conduction.
This review summarizes the identification of LRRC8 heteromers as VRAC components, and describes the similarities between LRRC8 protein and pannexins, and discusses whether VRAC performs greater osmotic pressure.
This article shows that LRRC8 channels are directly modulated by oxidation in a subunit-dependent manner.
LRRC8A Preparation Options
Membrane protein research has made significant progress over the past few years. Based on our versatile Magic™ membrane protein production platform, we could offer a range of membrane protein preparation services for worldwide customers in reconstitution forms and multiple active formats. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-LRRC8A antibody development services.
In the past years, Creative Biolabs has successfully produced many functional membrane proteins for our global customers. We are pleased to accelerate the development of our clients’ programs through our one-stop, custom-oriented service. For more detailed information, please feel free to contact us.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.