A comprehensive catalog of pre-validated antibodies, potent cytotoxic payloads, and diverse linker technologies, ready for your research and development needs.
Antibody-Drug Conjugate (ADC) Development Service
Are you currently facing challenges in developing highly specific and effective therapeutic agents with reduced off-target toxicity? Creative Biolabs' advanced Antibody-Drug Conjugate solutions help you accelerate drug discovery and obtain highly potent, targeted biopharmaceuticals through innovative conjugation chemistry and comprehensive validation platforms. We empower your projects with unparalleled precision, driving the next generation of targeted therapies.
Antibody-Drug Conjugates (ADCs)
Antibody-Drug Conjugates (ADCs) represent a sophisticated class of biopharmaceutical drugs designed for highly targeted therapy, primarily in cancer treatment. An antibody-drug conjugate constitutes a tripartite architecture featuring: a monoclonal antibody, a high-potency cytotoxin payload, and a molecular tether covalently joining both elements.
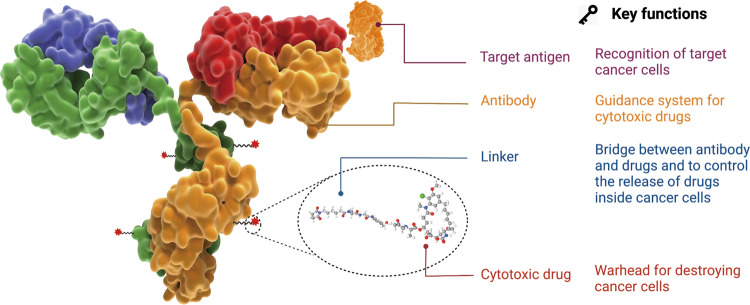 Fig.1 The structure and characteristic of an ADC drug.1,4
Fig.1 The structure and characteristic of an ADC drug.1,4
The monoclonal antibody serves as the targeting moiety, specifically recognizing and binding to a unique antigen highly expressed on the surface of target cells, such as cancer cells, while having limited expression on normal cells. This specificity is crucial for minimizing off-target toxicity. Upon binding, the ADC-antigen complex is typically internalized into the cell via receptor-mediated endocytosis.
The development of ADCs has evolved significantly since the first clinical trials in the 1980s. Early challenges included linker instability, heterogeneous drug-to-antibody ratios (DAR), and payload limitations. However, advancements in antibody engineering, the design of more stable and cleavable linkers, and the discovery of increasingly potent payloads have led to a new generation of highly effective ADCs. As of 2024, numerous ADCs have received regulatory approval for various cancers, and many more are in advanced stages of clinical development, highlighting their growing importance in precision medicine. The success of ADCs hinges on careful selection of the target antigen, the antibody's binding affinity, the payload's potency, and the linker's stability and cleavability, all contributing to a favorable therapeutic index.
Our Antibody-Drug Conjugates (ADCs) Solution
Creative Biolabs provides comprehensive Antibody-Drug Conjugate (ADC) solutions, tailored to your therapeutic needs. Understanding ADC classification, primarily based on linker and payload components, is crucial for optimizing efficacy and safety.
Based on Linker Chemistry:
- Cleavable Linkers: Designed for intracellular payload release, sensitive to specific conditions. Examples include peptide linkers (cleaved by lysosomal proteases like cathepsin B), hydrazone linkers (acid-labile in lysosomes), and disulfide linkers (reduced by intracellular glutathione).
- Non-Cleavable Linkers: Remain intact, with the antibody-linker-drug complex degrading in the lysosome for drug release. These offer stability but may have slower release kinetics.
Based on Conjugation Strategy:
- Lysine Conjugation: Generates heterogeneous ADCs exhibiting diverse drug-to-antibody ratios (DAR).
- Cysteine Conjugation: Utilizes engineered cysteine residues for more site-specific and homogeneous conjugation, leading to consistent DARs.
- Site-Specific Conjugation: Advanced techniques (e.g., enzymatic, unnatural amino acid incorporation, click chemistry) enable precise control over conjugation site and DAR, yielding highly homogeneous ADCs with improved pharmacokinetics and therapeutic index.
Creative Biolabs leverages expertise across all these ADC types, offering tailored design, synthesis, and characterization services to develop the most effective ADC for your specific therapeutic target and indication.
Contact Us About Bioconjugation Services
Application
Antibody-Drug Conjugates (ADCs) offer targeted delivery capabilities extending beyond oncology to diverse diseases requiring precise drug delivery.
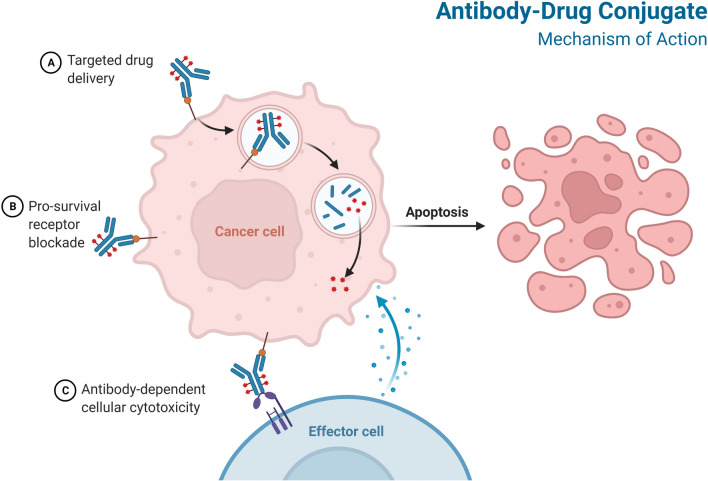 Fig.2 Mechanism of action of ADC.2,4
Fig.2 Mechanism of action of ADC.2,4
- Oncology: ADCs primarily target cancer cells with potent agents, minimizing systemic toxicity for various solid tumors and hematological malignancies, providing a viable therapeutic option.
- Autoimmune Diseases: Targeted delivery, including ADC-like constructs, shows promise for autoimmune diseases. By targeting specific immune cells or inflammatory mediators, therapeutics can be delivered precisely, offering focused treatment with reduced systemic immunosuppression.
- Infectious Diseases: Targeted delivery is also explored for infectious diseases, using antibodies to deliver antimicrobials directly to infected cells or microbes, potentially overcoming resistance.
- Other Therapeutic Areas: This principle extends to cardiovascular, fibrotic, and neurological disorders, enabling precise delivery to specific cells or tissues.
Creative Biolabs' expertise in conjugation and targeted delivery systems supports novel therapeutic strategies across these applications, leveraging precision medicine.
What We Can Offer?
Creative Biolabs is uniquely positioned at the forefront of targeted drug delivery innovation. Our team of expert biologists, chemists, and engineers brings over two decades of collective experience in developing sophisticated delivery solutions. We deliver an all-encompassing array of offerings engineered to fulfill your precise R&D requirements:
Ready-to-Use Conjugate Components
Customized Conjugation Services
Our bespoke service allows us to develop tailored ADCs from concept to validation, precisely meeting your project's unique specifications. This includes custom antibody modification, linker synthesis, and payload conjugation, as well as optimization of drug-to-antibody ratio (DAR) for specific therapeutic goals.
Ligand Conjugation Expertise
Specialized services in conjugating selected ligands to various delivery platforms, including nanoparticles, liposomes, and polymeric systems, extending beyond traditional ADCs.
Pre-Clinical Validation & Characterization
Comprehensive in vitro and in vivo testing to assess ADC targeting efficiency, cellular uptake, biodistribution, and therapeutic efficacy, ensuring robust data for your regulatory submissions.
Comprehensive Scientific Partnership
Partner with us to leverage our deep scientific knowledge, state-of-the-art facilities, and rigorous quality control for your targeted delivery projects, from experimental design to data analysis, providing end-to-end support.
Workflow

Why Choose Us?
Partnering with Creative Biolabs means choosing a path to accelerated drug development, enhanced therapeutic efficacy, and a significant reduction in off-target effects. Our commitment to innovation and scientific excellence ensures that your therapeutic agents reach their targets with unprecedented precision, unlocking new possibilities for disease treatment.
Unrivaled Expertise
Our team of highly specialized biologists, chemists, and engineers possesses profound scientific knowledge in antibody-drug conjugate design, synthesis, and characterization, built on decades of experience.
Cutting-Edge Technology
We leverage state-of-the-art platforms for antibody modification, linker synthesis, payload conjugation, and comprehensive in vitro and in vivo characterization, ensuring optimal ADC performance.
Tailored Customization & Flexibility
We offer highly customized ADC design and optimization services, adapting to your unique therapeutic goals and target profiles, providing unparalleled flexibility in your research.
Rigorous Quality & Reliability
Our unwavering commitment to scientific rigor ensures reliable, reproducible, and high-quality results for your critical projects, from raw material sourcing to final product validation.
Published Data
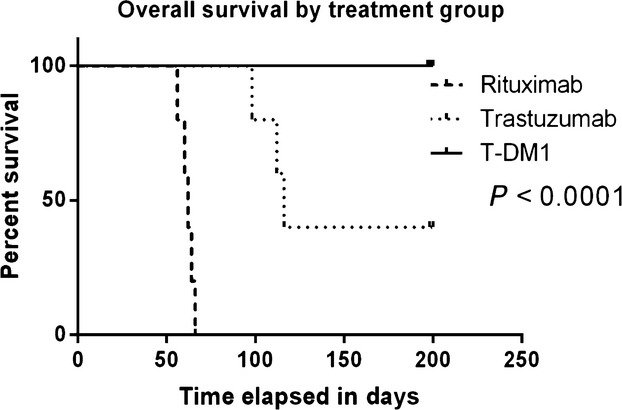 Fig.3 Overall survival in USC inoculated mice after treatment with weekly rituximab, T or T-DM1.3,4
Fig.3 Overall survival in USC inoculated mice after treatment with weekly rituximab, T or T-DM1.3,4
The ADC trastuzumab emtansine, or T-DM1, is a promising new therapeutic that combines the HER2-targeting antibody trastuzumab with a potent cytotoxic drug. This innovative approach functions as a highly specific delivery system, or "guided missile," that precisely delivers the chemotherapy payload directly to cancer cells that overexpress the HER2 protein, thereby maximizing its destructive potential while minimizing systemic toxicity to healthy tissues. The use of the trastuzumab component alone is already a standard treatment for HER2-positive breast and gastric cancers, where it works by binding to the HER2 receptor and inhibiting growth signals. Building on this success, a pivotal trial demonstrated the enhanced clinical activity of T-DM1 specifically in breast cancer patients who had developed resistance to trastuzumab, a population with limited treatment options. The trial's impressive results, which included a 43.6% objective response rate and a median progression-free survival of 9.6 months, highlighted T-DM1's potential to overcome established resistance mechanisms and provide a new therapeutic avenue. While these results showed significant promise, its application was not widely explored in other HER2-positive cancers at the time of this study. The article highlights that uterine serous carcinoma (USC), a highly aggressive subtype of endometrial cancer, is characterized by c-erbB2 amplification, which leads to HER2 protein overexpression. This genetic alteration is found in over 30% of USC cases and is associated with a particularly poor prognosis, making it a compelling new potential target for this ADC.
FAQs
Q: How do ADCs minimize side effects compared to traditional treatments?
A: ADCs deliver potent therapeutic agents directly to diseased cells by recognizing specific surface markers. This selective targeting minimizes healthy cell exposure, significantly reducing systemic side effects.
Q: What conditions can benefit from ADC technology?
A: Primarily developed for oncology, ADCs are also being explored for autoimmune disorders and certain infectious diseases, offering precise and effective treatment where specific cells or tissues are involved.
Q: Is ADC manufacturing reliable and scalable?
A: Advancements in bioconjugation chemistry and manufacturing have made ADC production highly reliable and scalable, ensuring consistent quality and meeting both research and clinical demands.
Q: How do ADCs compare to other emerging therapies?
A: ADCs uniquely combine biological targeting specificity with small molecule drug potency. They offer a focused approach, leading to higher drug concentrations at disease sites and reduced systemic toxicity, complementing other modalities.
Q: What are key considerations for designing a successful ADC?
A: Key considerations include selecting a highly specific target, a potent therapeutic agent, and a stable yet releasable linker. Optimizing these ensures efficient target delivery, effective payload release, and circulation stability for optimal outcomes.
Creative Biolabs is your trusted partner in Antibody-Drug Conjugates, offering unparalleled expertise and state-of-the-art solutions for ADC design, synthesis, and characterization. Our comprehensive services, from custom conjugation to rigorous preclinical validation, accelerate your drug discovery efforts, ensuring maximum precision and efficacy. We are committed to transforming targeted medicine with scientific excellence.
Connect with our experts for project-specific consultation and detailed insights.
References
- Fu, Zhiwen et al. "Antibody drug conjugate: the "biological missile" for targeted cancer therapy." Signal transduction and targeted therapy vol. 7,1 93. 22 Mar. 2022, DOI:10.1038/s41392-022-00947-7.
- Marei, Hany E et al. "Potential of antibody-drug conjugates (ADCs) for cancer therapy." Cancer cell international vol. 22,1 255. 13 Aug. 2022, DOI:10.1186/s12935-022-02679-8.
- English, Diana P et al. "T-DM1, a novel antibody-drug conjugate, is highly effective against primary HER2 overexpressing uterine serous carcinoma in vitro and in vivo." Cancer medicine vol. 3,5 (2014): 1256-65. DOI:10.1002/cam4.274.
- Distributed under Open Access license CC BY 4.0, without modification.



