Cell-Penetrating Peptide (CPP)-based delivery strategies: Mechanisms and Biomedical Applications
Cell-Penetrating Peptides (CPPs) have emerged as versatile and efficient delivery vectors in biomedicine, addressing the longstanding challenge of intracellular cargo transport. In this review, Creative Labs will take you through the mechanisms and biomedical applications of CPP-based delivery systems.
Introduction to Cell-Penetrating Peptides
What Are Cell-Penetrating Peptides (CPPs)?
Cell-Penetrating Peptides (CPPs), also known as protein transduction domains (PTDs), are short amino acid sequences (typically 5-30 residues) that exhibit exceptional cell membrane permeability and low inherent toxicity. By leveraging their biochemical properties, CPPs are capable of crossing biological membranes and transporting various molecules directly into cells. For example, cationic CPPs can promote interaction with negatively charged cell membranes, and amphipathic CPPs can enhance membrane permeability. There are many types of molecules they can carry, including small molecules, proteins, nucleic acids, and nanoparticles. During the drug delivery, CPPs are shown to improve the therapeutic efficacy by protecting the therapeutic cargos from enzymatic degradation in vivo, bypassing the plasma membrane's hydrophobic barrier, enhancing cargo bioavailability, and reducing off-target effects. As they possess these unique properties as carriers, CPPs are positioned at the heart of next-generation targeted drug delivery strategies. They are intensively investigated for potential applications in various fields, such as cancer immunotherapy, gene therapy, molecular imaging, and vaccine development.
At Creative Biolabs, we provide advanced CPP-based delivery technology services to support your translation of CPP innovations into practical biomedical solutions.
Classification of CPPs
The discovery of CPP can be traced back to the late 1980s, when researchers observed that certain viral proteins could enter cells spontaneously. As they exhibit high diversity in physicochemical properties and biological functions, CPP can be classified in three ways according to their origin, structure, and physicochemical properties (Figure 1). Of these classifications, the most common one is the classification according to physical-chemical properties, as it is closely related to CPPs' mechanism of membrane penetration and cargo delivery. The framework of this classification divides CPPs into three main types: cationic CPPs, amphipathic CPPs, and hydrophobic CPPs. They exhibit their unique functions due to their specific structures.
Cationic CPPs
Cationic CPPs are the most extensively studied subclass of CPPs. They are characterized by a high content of positively charged amino acids (e.g., arginine, lysine) in their sequences. Therefore, under physiological pH conditions, cationic CPPs are positively charged, which enables them to interact electrostatically with the negatively charged components of cell membranes (e.g., carboxyl and phosphate groups), facilitating non-receptor-mediated cellular uptake. A typical example of this is the HIV-derived TAT peptide (sequence: RKKRRQRRR), which can efficiently deliver cargoes, such as proteins, nucleic acids, and nanoparticles, into cells. It has been found that the number and spatial arrangement of cationic residues (e.g., arginine) in CPPs could directly influence their cellular uptake efficiency. For instance, polyarginine R8/R9 exhibits higher transmembrane ability than shorter polyarginine chains. Furthermore, some cationic CPPs (e.g., nuclear localization signal, NLS) are found to be able to target cell nuclei by utilizing classical nuclear import pathways. This unique function further expands the CPP application in gene therapy.
Amphipathic CPPs
Amphipathic CPPs account for over 40% of known CPPs. They are characterized by segregated polar (cationic) and nonpolar (hydrophobic) regions in their structure. The amphipathic feature of this type often facilitates membrane penetration by interacting with both the hydrophilic head groups and the hydrophobic core of cell membranes. During the membrane interaction, amphipathic CPPs usually adopt α-helical or β-sheet conformations to insert into lipid bilayers. α-helical amphipathic CPPs (e.g., MAP) insert into lipid monolayers via hydrophobic regions, while β-sheet variants (e.g., some MPG analogs) are more sensitive to membrane charge. Moreover, this subclass excels at delivering large cargoes (e.g., antibodies, plasmid DNA), and they are currently often modified with targeting ligands (e.g., folate, RGD) to enhance tissue specificity.
Hydrophobic CPPs
Hydrophobic CPPs are the least common type of CPP. They are primarily composed of nonpolar amino acids (e.g., alanine, leucine) with minimal positive charge (<20% of the sequence). Their membrane penetration relies on hydrophobic interactions with the lipid bilayer of the cell membrane, via direct translocation or inverted-micelle mechanisms. The origin of this subclass could be natural (e.g., C105Y) or synthetic (e.g., stapled peptides). Compared with the other two subclasses, hydrophobic CPPs exhibit relatively low cytotoxicity. However, they require careful sequence optimization to avoid nonspecific cell membrane disruption. Currently, they are mainly used for delivering hydrophobic cargoes (e.g., small-molecule drugs, peptide toxins) and targeting intracellular organelles such as mitochondria.
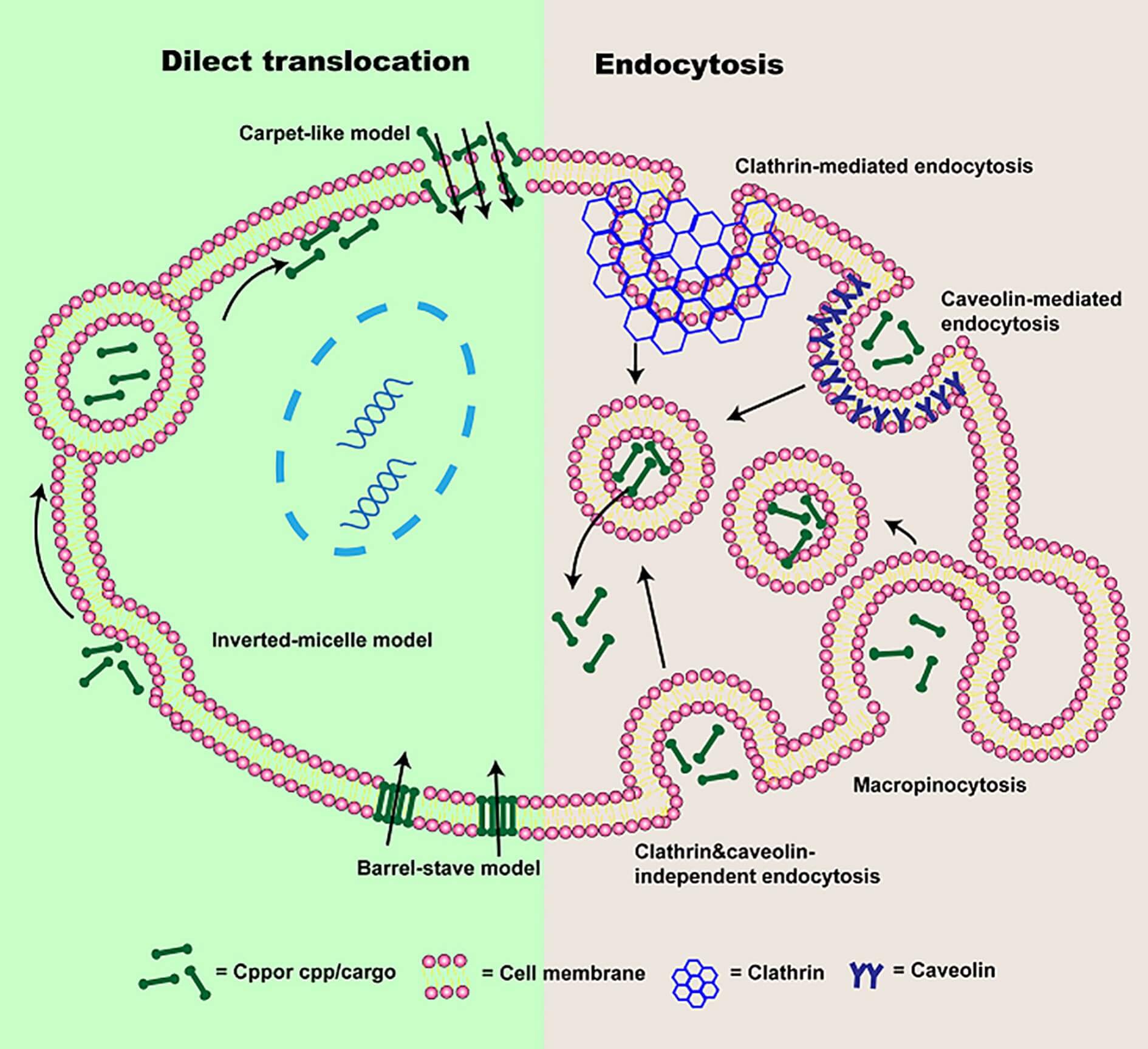 Fig.1
Types of CPPs based on origin, conformation, or physical-chemical properties2.
Fig.1
Types of CPPs based on origin, conformation, or physical-chemical properties2.
How Do CPPs Work?
The mechanisms of the CPP uptake depend on multiple factors, including peptide sequence (e.g., cationicity, amphiphilicity), cargo type (e.g., peptides, proteins, nucleic acids), and the physicochemical properties of target cell membranes. CPPs utilize two primary pathways for internalization (Figure 2) and one pathway for cargo release.
Direct Translocation
Direct translocation is an energy-free process. The CPPs are inserted into the cell membrane through transient pore formation or inverted micelle structure without cellular energy (e.g., ATP) or involvement of membrane proteins. Cationic CPP-cargo complexes (e.g., R9, cyclic arginine-rich peptides) can cross the bilayer directly through the transient membrane perturbations (e.g., lipid rearrangement or pore formation), which are induced by their electrostatic interaction with negatively charged membrane components (e.g., phosphatidylserine). Amphipathic CPPs, such as melittin, can cross the membrane by utilizing their cationic and hydrophobic regions. Their cationic region could bind to the membrane headgroups, and their α-helical conformations could insert the hydrophobic regions into the lipid core. This pathway is typically used for the efficient intracellular delivery of small cargoes, such as short peptides.
Endocytosis
Endocytosis is the most common mechanism for the uptake of CPP. It relies on cellular energy and membrane trafficking. According to the CPP type and cell line, it can be further divided into several subtypes of pathways, including clathrin-mediated endocytosis, caveolin-mediated endocytosis, and macropinocytosis. Cationic CPPs (such as TAT) commonly induce clathrin-mediated endocytosis by binding to Heparan sulfate proteoglycans (HSPGs) on the membrane, leading to vesicle formation. Amphipathic CPPs (such as penetratin) may target lipid rafts on the cell membrane and depend on caveolin-mediated endocytosis. Although this pathway is useful for the internalization of large cargoes (e.g., antibodies, nanoparticles), it also carries the risk of trapping CPP-cargo complexes in endosomes, which could result in limited therapeutic efficacy.
Cargo Release
Cargo release is critical for CPPs to deliver active molecules to intracellular targets. Following endocytosis, the drug's effectiveness depends on its ability to escape from endosomes. CPP-cargo complexes must escape endosomes before lysosomal degradation. Hence, some CPPs are designed to contain pH-sensitive sequences or fusogenic domains to facilitate cytosolic release. For instance, TAT-conjugated superoxide dismutase (TAT-SOD) has been shown to disrupt endosomal membranes by leveraging the acidic endosomal pH (5.0-6.0) to induce a conformational change through the cationic residues in the TAT peptide. Amphipathic CPPs, such as MPG, can also utilize hydrophobic interactions to destabilize the lipid bilayer of endosomes. In terms of nuclear targeting after cytosolic release, CPPs with nuclear localization signals (NLS), such as NrTP6 (a crotamine mimic), can bind to importins, facilitating nuclear translocation.
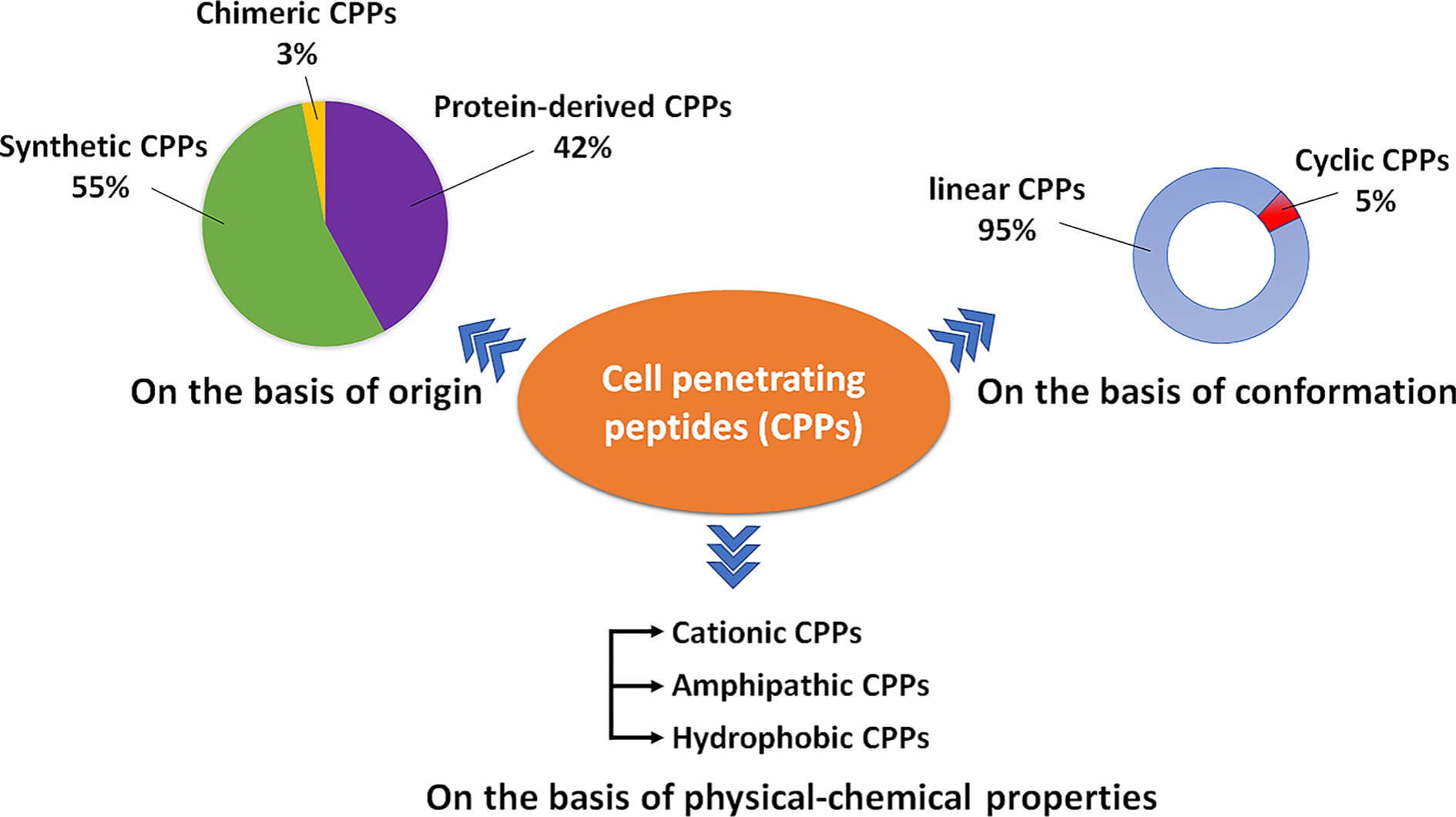 Fig.2 The cellular
uptake mechanisms of CPPs2.
Fig.2 The cellular
uptake mechanisms of CPPs2.
Advantages of CPP-based delivery systems
CPP-based delivery systems offer five key benefits that make them attractive for biomedical applications (Table 1).
Versatility
Description: CPPs can transport diverse cargos, including proteins, peptides, DNA, RNA, and nanoparticles.
Structural cause: The cationic/hydrophobic regions could enable CPPs to form stable complexes with cargoes via electrostatic or hydrophobic interactions.
Efficiency
Description: CPPs can facilitate high intracellular delivery efficiency without needing external agents.
Structural cause: CPPs can leverage their cationic or hydrophobic regions to interact with specific components of cell membranes.
Biocompatibility
Description: Compared with other transfection methods, CPPs possess minimal cytotoxicity and immunogenicity.
Structural cause: As CPPs are inherently non-toxic short peptides, they have low immunogenicity and no cytotoxicity. Moreover, CPPs can be modified to reduce the side effects of the therapeutic cargos.
Targeting Potential
Description: CPPs can be modified to target cell types, including CHO cells, tumor cells, or even organelles.
Structural cause: CPPs can be conjugated with targeting ligands to improve their cell-type specificity.
Scalability
Description: CPP production is available for industrial scalability.
Structural cause: Short sequences of CPPs can be produced by straightforward solid-phase synthesis. Synthetic CPPs (e.g., cyclic CPPs, D-form CPPs) can be produced with high purity and consistent quality.
Biomedical Applications of CPPs
Cell-penetrating peptides (CPPs) are widely applied in many aspects of biomedical applications due to their superior membrane permeability and cargo compatibility.
Drug Delivery
CPPs have revolutionized the delivery of therapeutic molecules, especially for drugs that cannot easily cross membranes. In the field of immunotherapy, CPPs can be loaded with various molecules, such as small molecules, proteins, and peptides, to improve their cellular uptake, in vivo stability, or both. For example, TAT-conjugated insulin exhibits 7-fold higher intestinal absorption compared to free insulin. Cyclic CPP is proven to enhance transdermal insulin delivery through improved proteolytic stability. Moreover, by conjugation with R9, superoxide dismutase (SOD) can inhibit the hepatoma cell proliferation via reducing their intracellular ROS.
Gene and siRNA Delivery
CPPs can be used for gene silencing and editing by delivering nucleic acids (DNA, siRNA, mRNA) efficiently. For instance, cyclic amphipathic CAPs can efficiently deliver siRNA intracellularly, knocking out the target gene with high efficiency via clathrin- and caveolin-mediated endocytosis. CPPs could also be integrated with nanoparticles for enhanced stability and transfection rates. A typical example is the use of CPP-modified PLGA nanoparticles for delivering p53-encoding DNA into cancer cells, which enables the activation of tumor suppressor pathways.
Imaging and Diagnostics
Fluorescently labeled CPPs can be applied for cell imaging, tumor localization, and biomarker tracking. By conjugation with imaging agents, CPPs enable precise detection of disease-related changes at the molecular level. For example, CPP-functionalized gold nanoparticles are used for in vivo tumor imaging. Additionally, CPP-fluorescent dye conjugates can enhance diagnostic accuracy by tracking the distribution of intracellular cargo.
Vaccine Development
As antigen carriers, CPPs contribute to vaccine development in many ways. Firstly, they can boost antigen uptake and immune responses. In HPV therapy, CPPs can induce long-term anti-tumor immunity by efficiently delivering the E7 antigen to immune cells. They are also proven to improve the antigen presentation of dendritic cells, which is critical for vaccine efficacy.
Blood–Brain Barrier (BBB) Penetration
As CPPs can cross the blood-brain barrier (BBB), they can deliver neurotherapeutic agents to the central nervous system (CNS) to treat conditions such as Parkinson's and Alzheimer's diseases. For instance, an apamin analog has been found to penetrate the BBB without causing neurotoxicity. It is considered a promising CPP carrier for delivering neurotherapeutic drugs.
Challenges and Future Directions
Despite remarkable progress, several challenges remain in the CPP application, including poor serum stability, low tumor targeting, nonspecific toxicity, and difficulties in clinical translation. These challenges are intensely addressed and are expected to be a focus of future research (Table 1).
Table 1: Challenges and Future Directions of CPP Application.
| Key Challenges | Emerging Trend/Future Direction |
|---|---|
| Poor Serum Stability | Structural modification to enhance proteolytic resistance |
| Low Tumor Targeting | Development of stimuli-responsive CPPs that can be triggered by the tumor microenvironment. |
| Development of tumor-targeting ligand-conjugated CPPs | |
| Nonspecific Toxicity | Optimization of the CPP structure, such as glycosylation, to weaken the non-target cell interaction |
| Optimization of CPP delivery systems, such as fusion with endosomolytic peptides to avoid membrane damage | |
| Clinical Translation Difficulties | Improve CPP formulation scalability & reproducibility |
| Clarify the in vivo pharmacokinetics of CPPs |
Related Services You May Be Interested in
FAQs
What are cell-penetrating peptides?
Cell-penetrating peptides (CPPs) are short peptides (5-30 amino acids) (e.g., TAT, penetratin) that exhibit enhanced membrane permeability. They can deliver various cargoes into cells via endocytosis or direct translocation.
How do cell-penetrating peptides work?
-
There are two pathways for CPPs to deliver cargos intracellularly, and one pathway for CPPs to
release cargos.
(1) Endocytosis: Cationic/amphipathic CPPs can bind to membrane components (e.g., HSPGs, lipid rafts), thereby triggering clathrin- and caveolin-mediated endocytosis.
(2) Direct translocation: Hydrophobic/cationic CPPs can induce transient membrane perturbations, allowing them to cross bilayers directly.
(3) Endosomal escape: CPPs could release the cargos into the cytosol by disrupting endosomal membranes. - CPPs may also enhance the in vivo stability of therapeutic agents, thereby improving the drug efficacy.
Conclusion
Cell-penetrating peptides are reshaping the landscape of drug and gene delivery by enabling efficient, targeted, and safe intracellular access. With continuous investigation into its potential in biomedical applications, it is expected that CPPs will play a vital role in the efficient delivery of therapeutic drugs in clinical biomedicine.
With years of experience, Creative Biolabs provides end-to-end support for CPP discovery, modification, and delivery system development, helping our clients to accelerate their research progress. For more customized CPP-based delivery information, please visit Creative Biolabs' Targeted Delivery Service and make an appointment with our experts to bring your next therapeutic innovation to life.
References
- Zhang, H. et al. "Recent Advances of Cell-Penetrating Peptides and Their Application as Vectors for Delivery of Peptide and Protein-Based Cargo Molecules." Pharmaceutics 15, 2093 (2023). https://www.mdpi.com/1999-4923/15/8/2093
- Xie, J. et al. "Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application." Front. Pharmacol. 11, 697 (2020). https://www.frontiersin.org/article/10.3389/fphar.2020.00697/full Distributed under Open Access license CC BY 4.0, without modification.
- Nam, S. H., Park, J. & Koo, H. "Recent advances in selective and targeted drug/gene delivery systems using cell-penetrating peptides." Arch. Pharm. Res. 46, 18–34 (2023). https://link.springer.com/10.1007/s12272-022-01425-y
- Hasannejad-Asl, B., Pooresmaeil, F., Takamoli, S., Dabiri, M. & Bolhassani, A. "Cell penetrating peptide: A potent delivery system in vaccine development." Front. Pharmacol. 13, 1072685 (2022). https://www.frontiersin.org/articles/10.3389/fphar.2022.1072685/full
Created in September 2025



