We provide tailored services to discover and optimize novel antibody fragments with high specificity and internalization rates, ensuring the ideal foundation for your FDC.
Antibody Fragment-Drug Conjugate (FDC) Development Service
Are you currently facing challenges such as poor tumor penetration with conventional ADCs, long development cycles, or off-target toxicities? Creative Biolabs' FDC development services help you accelerate drug discovery and enhance therapeutic efficacy by leveraging our advanced protein engineering and conjugation technologies. Our bespoke solutions ensure your therapeutic agents reach their intended targets with unprecedented precision, unlocking new possibilities for disease treatment.
Antibody Fragment-Drug Conjugates (FDC)
Antibody-Drug Conjugates (ADCs) have transformed precision oncology by integrating antibody specificity with cytoreductive agent potency. However, the large size of full-length antibodies can limit their ability to penetrate dense, solid tumors, and the Fc portion can sometimes lead to unwanted off-target effects. This has led to the development of a new generation of targeted therapeutics: Antibody Fragment-Drug Conjugates (FDCs). FDCs utilize smaller antibody fragments, such as single-chain variable fragments (scFv) or Fab fragments, which retain the specific antigen-binding capabilities of the full antibody but are significantly smaller. This reduced size allows for enhanced tumor penetration and faster clearance from the bloodstream, minimizing systemic toxicity. For both ADCs and FDCs, the efficacy hinges on the antibody's ability to bind to the target antigen on the cancer cell and, crucially, to be efficiently internalized (endocytosed) by the cell to deliver the cytotoxic payload. The internalized conjugate is then trafficked to the lysosome, where the payload is released to kill the cell.
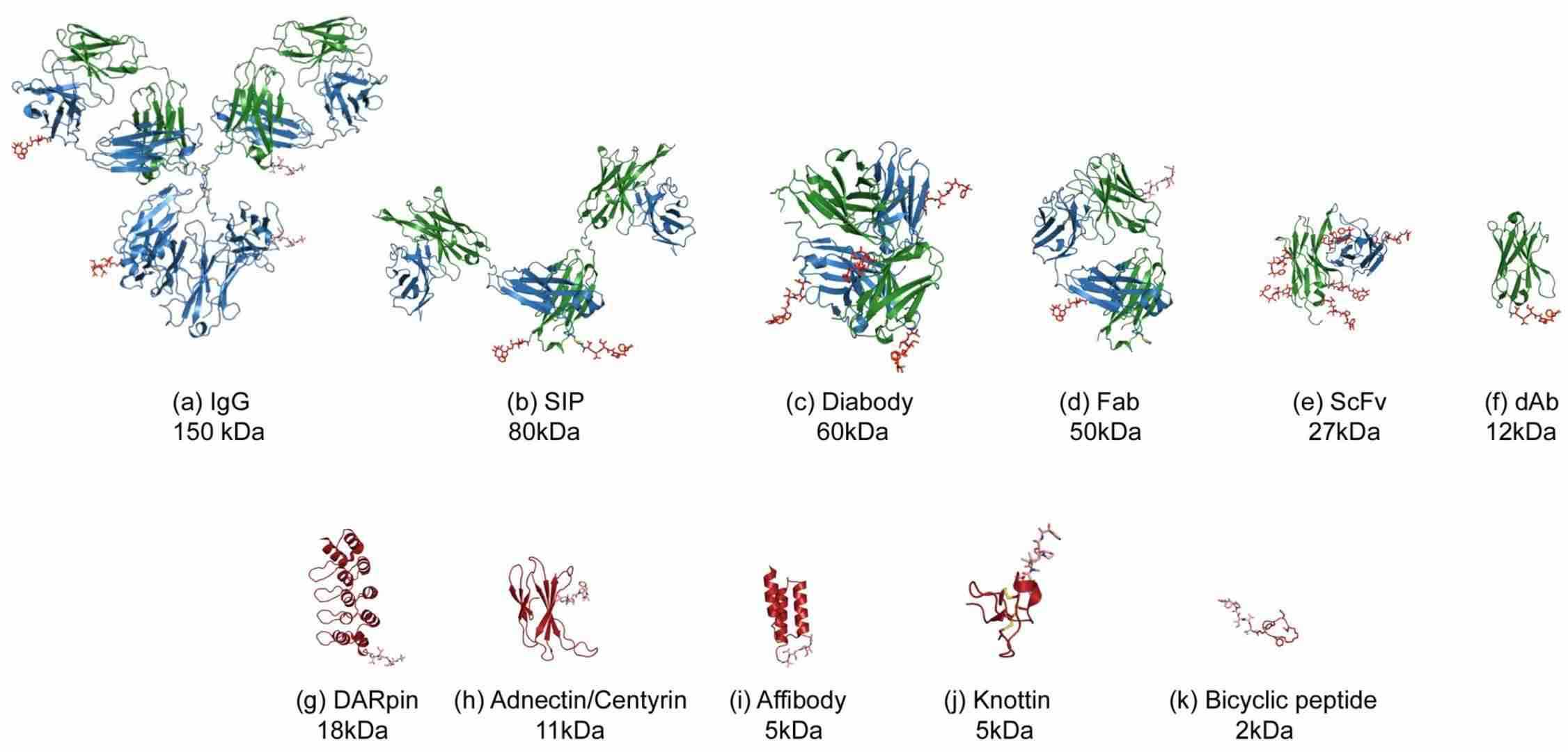 Fig.1 Diverse configurations for Antibody (Fragment) or substitute molecular scaffold–drug conjugates.1,3
Fig.1 Diverse configurations for Antibody (Fragment) or substitute molecular scaffold–drug conjugates.1,3
Our Antibody Fragment-Drug conjugates (FDC) Solution
The design and creation of FDCs represent a sophisticated solution for targeted drug delivery. Various types of antibody fragments can be used, each with unique properties. Single-chain variable fragments (scFvs) constitute minimal binding units, containing tethered variable heavy/light chains. They enable superior neoplasm infiltration yet exhibit monovalency, potentially diminishing avidity. Fab fragments possess greater mass and bivalency, conferring enhanced target engagement. The choice of fragment depends on the therapeutic application. A critical aspect of FDC development is the conjugation strategy, which determines the drug-to-antibody ratio (DAR) and the stability of the conjugate. Innovative techniques, such as site-specific conjugation using engineered cysteine residues (e.g., Intra-Domain Cysteines or IDC), allow for precise control over the DAR and attachment site. This approach yields a more homogeneous product with predictable properties, improved stability, and reduced immunogenicity, which is vital for the safety and efficacy of the final therapeutic.
Application
The unique properties of FDCs make them highly suitable for a range of therapeutic applications, particularly in areas where traditional ADCs face limitations. Key applications include:
- Superior Tumor Penetration: The smaller size of FDCs is a critical advantage, allowing them to navigate the dense extracellular matrix and overcome the high interstitial pressure found in solid tumors. This enhanced penetration ensures that a greater concentration of the therapeutic payload reaches the core of the tumor, improving efficacy.
- Reduced Off-Target Toxicity: By virtue of their faster systemic clearance from the body, FDCs create a wider therapeutic window. This means the powerful cytotoxic drug is present in the bloodstream for a shorter period, significantly reducing exposure to healthy organs and minimizing the risk of dose-limiting side effects.
- Versatile Payload Delivery: FDCs are not limited to delivering only cytotoxic drugs. Their adaptable nature allows them to carry a diverse range of therapeutic payloads, such as immunomodulatory agents to re-engage the immune system, gene-editing tools for precision genome modification, or even radioisotopes for targeted radiotherapy.
- Broad Therapeutic Potential: The precision targeting and flexible payload capacity of FDCs enable their application across a wide spectrum of diseases. This includes not only various types of cancers but also autoimmune disorders where specific immune cells need to be targeted, and infectious diseases requiring localized delivery of antimicrobial agents.
Contact Us About Bioconjugation Services
What We Can Offer?
Creative Biolabs is uniquely positioned at the forefront of targeted drug delivery innovation. Our team of expert biologists, chemists, and engineers brings over two decades of collective experience in developing sophisticated delivery solutions. We offer a comprehensive suite of products and services to empower your research and development needs.
Customized Discovery & Optimization
Ready-to-Use FDC Building Blocks
Our catalog includes a selection of pre-validated antibody fragments and linker-payload systems ready for rapid integration into your research.
Expert Conjugation Services
We specialize in conjugating selected cytotoxic payloads to various antibody fragments using advanced linker technologies, ensuring stability and controlled drug release.
Preclinical Validation
Our comprehensive validation services include in vitro assays to assess targeting efficiency, cellular uptake, and cytotoxicity, as well as in vivo studies to evaluate biodistribution and therapeutic efficacy.
Scientific Partnership & Support
Collaborate with our deep scientific team and leverage our state-of-the-art facilities and rigorous quality control for your targeted delivery projects, from experimental design to data analysis.
Workflow

Why Choose Us?
Collaborating with Creative Biolabs selects an expedited therapeutic development trajectory, enhanced therapeutic performance, and mitigated off-target consequences. Our dedication to pioneering solutions and research superiority guarantees your agents achieve target engagement with unmatched accuracy.
Demonstrated Proficiency
Our cohort of uncommonly specialized biologists, chemists, and engineers commands profound technical mastery in antibody fragment engineering and bioconjugation science.
Advanced Methodologies
We utilize cutting-edge systems for fragment fabrication, linker architecture, and site-directed conjugation, facilitating exceptionally monodispersed and stable FDC generation.
Augmented Potency & Tissue Permeation
Our FDCs exhibit heightened solid tumor infiltration versus traditional ADCs due to reduced dimensions, yielding amplified therapeutic payload deposition at disease loci.
Exacting Standards Assurance
Our adherence to methodological exactitude and stringent quality assurance protocols delivers replicable outcomes and premium-grade data for pivotal initiatives. Peer-reviewed evidence and validated internal achievements persistently substantiate our methodology's dependability.
Published Data
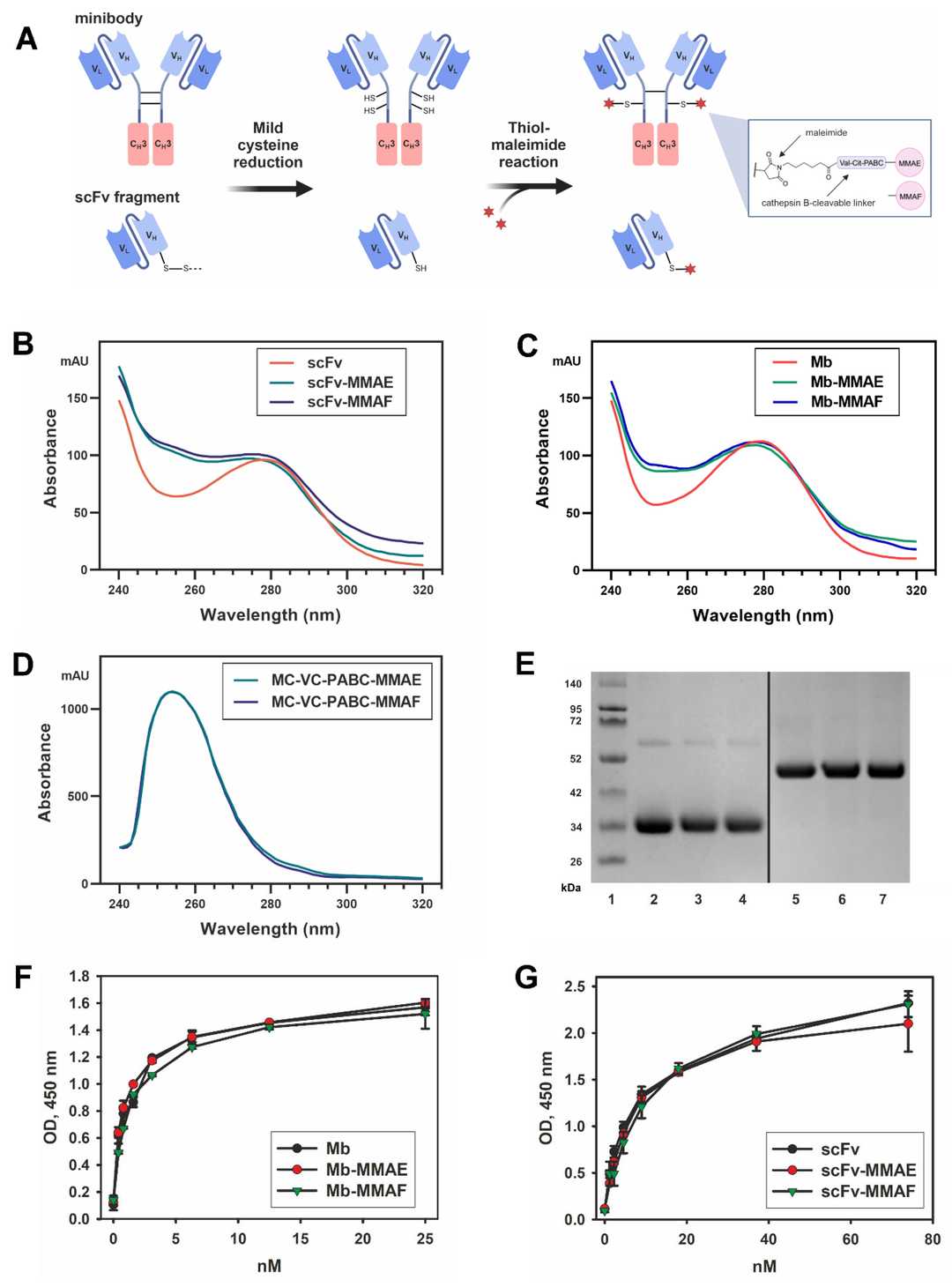 Fig.2 Generation of FDCs and characterization of their antigen-interaction characteristics.2,3
Fig.2 Generation of FDCs and characterization of their antigen-interaction characteristics.2,3
The study's experimental design focused on synthesizing and evaluating two types of Antibody Fragment-Drug Conjugates (FDCs)—minibody-based and scFv-based—to target GD2-positive tumor cells. Dual FDC variants were functionalized with either monomethyl auristatin E (MMAE) or monomethyl auristatin F (MMAF). The researchers used various GD2-positive human tumor cell lines, including neuroblastoma, melanoma, and osteosarcoma, to test the FDCs' efficacy in vitro.
The results demonstrated that both minibody-based and scFv-based FDCs successfully inhibited tumor cell proliferation. However, the minibody-based FDCs consistently showed a more pronounced cytotoxic effect across all tested cell lines, particularly when conjugated with MMAE. This enhanced potency was quantified through the half-maximal inhibitory concentration (IC50) values, which were significantly lower for the minibody-based FDCs. The mechanism of action was also investigated, revealing that the FDCs caused cell cycle arrest in the G2 phase, leading to apoptosis. The increased effectiveness of the minibody-based FDCs was attributed to their bivalent antigen binding, which provided a stronger interaction with the target cells compared to the monovalent binding of the scFv fragments, as well as their higher drug-to-antibody ratio. The data suggests that optimizing the FDC structure to increase both antigen valency and drug load could be a promising strategy for developing more effective anti-tumor therapies.
FAQs
Q: How does the smaller size of an FDC impact its therapeutic potential compared to a full-length ADC?
A: The smaller size of an FDC allows it to penetrate dense tumor tissue more effectively, delivering the therapeutic payload directly to the cancer cells. This can lead to a stronger on-site effect, while faster clearance from the body helps to minimize systemic exposure and potential side effects.
Q: What are the main factors to consider when selecting an antibody fragment for an FDC?
A: Key factors include the fragment's binding affinity, its specificity for the target antigen, and its internalization rate. The valency of the fragment (monovalent scFv vs. bivalent Fab) is also a crucial consideration, as it impacts both binding strength and conjugation strategy.
Q: Is the stability of the linker between the fragment and the drug a concern for FDCs?
A: Yes, linker stability is paramount. A robust molecular bridge is essential to avert preemptive payload liberation in circulation, preventing systemic collateral damage. The linker must be engineered to remain intact until the FDC is internalized by the target cell, where a specific mechanism (e.g., pH-sensitive or protease-cleavable) triggers the release of the payload.
Q: How do you ensure the FDC will only target cancer cells and not healthy cells?
A: Targeting specificity is achieved by selecting an antibody fragment that binds exclusively to an antigen overexpressed on the surface of cancer cells. Comprehensive screening and in vitro validation against both target-positive and target-negative cell lines are essential to confirm the conjugate's selective cytotoxicity.
Q: What are the primary advantages of site-specific conjugation methods for FDCs?
A: Site-specific conjugation produces a highly homogeneous FDC with a well-defined drug-to-antibody ratio (DAR). This consistency is critical for reproducible results and predictable pharmacokinetics. It can also help maintain the fragment's binding affinity, which can be compromised by random conjugation methods.
At Creative Biolabs, we enable your scientific exploration through innovative FDC technological solutions. We are committed to providing the highest quality products and services to help you achieve your therapeutic goals.
Connect with our experts for project-specific consultation and detailed insights.
References
- Deonarain, Mahendra P et al. "Small-Format Drug Conjugates: A Viable Alternative to ADCs for Solid Tumours?." Antibodies (Basel, Switzerland) vol. 7,2 16. 31 Mar. 2018, https://doi.org/10.3390/antib7020016
- Kalinovsky, Daniel V et al. "Minibody-Based and scFv-Based Antibody Fragment-Drug Conjugates Selectively Eliminate GD2-Positive Tumor Cells." International journal of molecular sciences vol. 24,2 1239. 8 Jan. 2023, https://doi.org/10.3390/ijms24021239
- Distributed under Open Access license CC BY 4.0, without modification.



