Peptide-Drug Conjugate (PDC) Development Service
Are you currently facing challenges with systemic toxicity, poor drug bioavailability, or insufficient cellular uptake in your biopharmaceutical projects? Creative Biolabs' Peptide-Drug Conjugates (PDCs) can help you enhance drug targeting and improve therapeutic outcomes through our advanced peptide engineering and conjugation technologies. Our solutions are designed to deliver potent payloads directly to the disease site, minimizing off-target effects and unlocking the full potential of your therapeutic agents.
Peptide-Drug Conjugates (PDCs)
Peptide-Drug Conjugates (PDCs) are a class of targeted therapeutic agents designed to deliver a potent cytotoxic drug selectively to a specific cell type, most notably cancer cells. This innovative approach aims to increase the efficacy of the drug while minimizing systemic toxicity, a common drawback of traditional chemotherapy. A PDC is composed of three main components: a homing peptide that recognizes a specific biomarker on the target cell, a potent payload drug to destroy the cell, and a linker that connects the two. This modular design allows for the precise delivery of the therapeutic agent directly to the intended site of action, improving the therapeutic window.
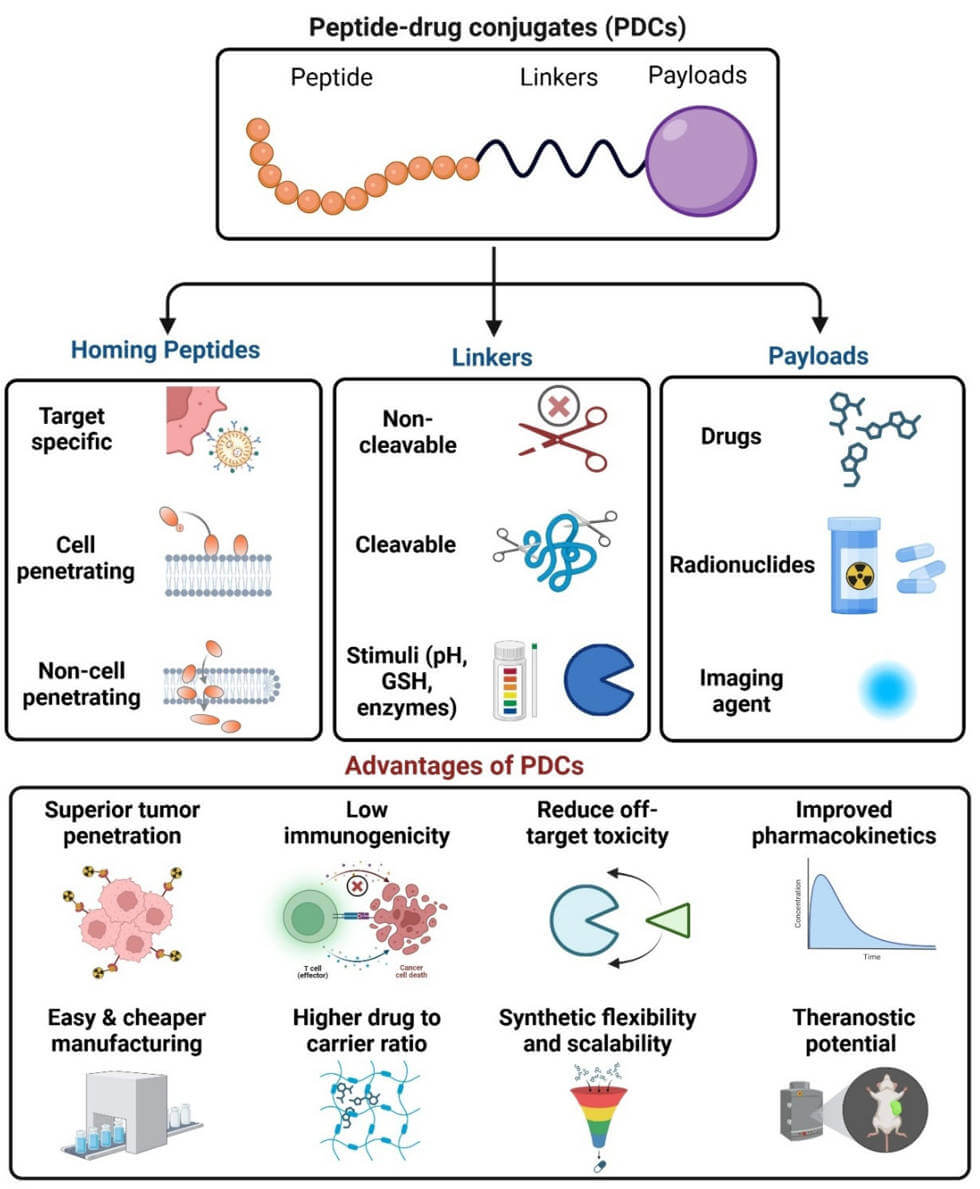 Fig.1 Peptide–drug conjugate (PDC).1,4
Fig.1 Peptide–drug conjugate (PDC).1,4
Key Components of PDCs:
This component is a short chain of amino acids that acts as a targeting vehicle. It is designed to bind with high affinity to receptors or antigens that are overexpressed on the surface of the target cell. By recognizing these specific biomarkers, the homing peptide ensures the PDC is internalized by the correct cells, leaving healthy cells largely unharmed. An example includes peptides that target receptors like the somatostatin receptor or integrins.
Peptide-Drug Conjugates (PDCs) Solutions
Our PDC solutions are highly customizable and can be tailored to various therapeutic needs. We offer different types of PDCs based on their core components and intended function:
- Targeting PDCs: These conjugates are designed to accumulate at a disease site by leveraging the high affinity of a specific peptide for a receptor that is overexpressed on the target cell. This provides a direct and efficient way to deliver the drug payload.
- Dual-Action PDCs: We can engineer PDCs that incorporate a peptide with a therapeutic effect of its own, in addition to carrying a cytotoxic payload. This dual-action mechanism provides a synergistic effect, enhancing the overall efficacy of the treatment.
- Stimuli-Responsive PDCs: We engineer PDCs with linkers that are sensitive to specific stimuli present at the disease site, such as low pH, specific enzymes, or hypoxia. This ensures that the drug is only released upon reaching its target, maximizing efficacy and minimizing off-target effects.
Contact Us About Bioconjugation Services
Application
Peptide-Drug Conjugates have a wide range of applications in various therapeutic areas where targeted and efficient drug delivery is critical. These include:
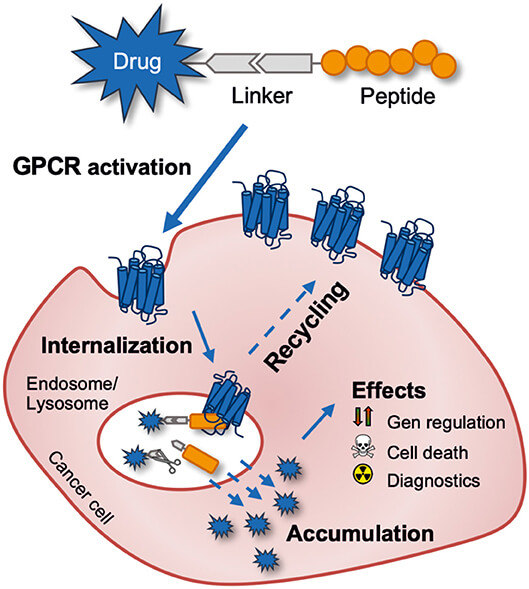 Fig.2 Targeting a tumor-expressed G protein-coupled receptor for anti-cancer drug delivery with a peptide-drug conjugate.2,4
Fig.2 Targeting a tumor-expressed G protein-coupled receptor for anti-cancer drug delivery with a peptide-drug conjugate.2,4
- Oncology: PDCs can deliver potent chemotherapeutic agents directly to tumor cells, reducing systemic toxicity and potentially overcoming multi-drug resistance.
- Infectious Diseases: By improving the bioavailability of antiviral or antibacterial drugs, PDCs can enhance their effectiveness against pathogens while minimizing side effects.
- Inflammatory Diseases: PDCs can be designed to target inflammatory cells, delivering anti-inflammatory agents directly to the site of inflammation.
- Neurological Disorders: The small size of PDCs allows them to potentially cross the blood-brain barrier, offering a promising avenue for treating central nervous system disorders.
- Genetic Disorders: PDCs can be employed in the delivery of nucleic acid therapeutics like small interfering RNA (siRNA) and mRNA, providing a solution to the challenges of delivering these sensitive molecules to cells.
What We Can Offer?
Creative Biolabs is uniquely positioned at the forefront of targeted drug delivery innovation. Our team of expert biologists, chemists, and engineers brings decades of collective experience in developing sophisticated delivery solutions. We supply a spectrum of solutions customized for your precise R&D requirements:
- Custom Peptide Design and Synthesis: We offer bespoke services to design and synthesize novel peptides and PDCs from concept to validation, precisely meeting your project's unique specifications. This includes custom peptide modification, drug-linker selection, and conjugation optimization.
- Comprehensive Product Catalog: A selection of pre-formulated delivery systems and a catalog of validated peptides, linkers, and targeted modules are available for your research and development needs.
- Conjugation Services: Our expertise lies in conjugating selected drugs to various peptide platforms. We ensure precise and stable conjugation to maintain the integrity and function of the therapeutic agent.
- Pre-Clinical Validation: We conduct rigorous in vitro and in vivo testing to assess targeting efficiency, cellular uptake, biodistribution, and therapeutic efficacy, providing you with the data you need to confidently advance your project.
- Scientific Support: Partner with us to leverage our deep scientific knowledge, state-of-the-art facilities, and rigorous quality control for your targeted delivery projects, from experimental design to data analysis.
Workflow

Why Choose Us?
Partnering with Creative Biolabs means choosing a path to accelerated drug development, enhanced therapeutic efficacy, and a significant reduction in off-target effects. Our commitment to innovation and scientific excellence ensures that your therapeutic agents reach their targets with unprecedented precision, unlocking new possibilities for disease treatment.
Proven Expertise
Our team of highly specialized biologists, chemists, and engineers possesses deep scientific knowledge in PDC systems and targeting module development. We have a track record of successfully navigating the complexities of targeted delivery.
Innovative Technology
We leverage state-of-the-art platforms for peptide synthesis, conjugation, and characterization. Our cutting-edge methodologies enable the creation of stable, highly effective conjugates with superior performance.
Tailored Customization & Flexibility
We offer customized peptide and linker design, allowing for the precise optimization of your therapeutic goals and target cell types.
Rigorous Quality & Reliability
Our commitment to scientific rigor ensures reliable, reproducible, and high-quality results for your critical projects. We adhere to stringent quality control standards at every stage of the process.
Published Data
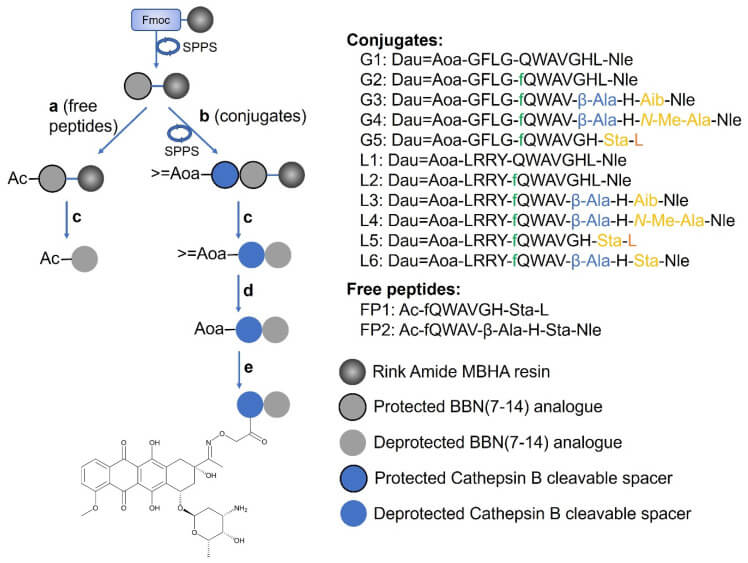 Fig.3 Fabrication of Dau-BBN (7-14) conjugates and BBN (7-14) analogues as unbound peptide molecular assemblies.3,4
Fig.3 Fabrication of Dau-BBN (7-14) conjugates and BBN (7-14) analogues as unbound peptide molecular assemblies.3,4
The study details the development of new peptide-drug conjugates (PDCs) based on the bombesin peptide, designed to target the gastrin-releasing peptide receptor (GRP-R) for cancer therapy. GRP-R is a highly expressed protein in various cancers, making it an excellent target for selective drug delivery. The researchers created eleven PDCs by linking bombesin analogues to the chemotherapy drug daunorubicin. The study evaluated these conjugates in both lab (in vitro) and animal (in vivo) models to test their effectiveness and safety against prostate and breast cancer. In vitro, the researchers used MTT assays to measure the anti-proliferative effects and calculate IC50 values on three human cancer cell lines (MDA-MB-231, MDA-MB-453, and PC-3) and one healthy cell line (MRC-5) to check for toxicity. Flow cytometry was used to quantify cellular uptake, and confocal microscopy allowed for the visualization of the conjugates' localization within the cells. The results showed that two PDCs, L5 and L6, had remarkable anti-proliferative activity and were efficiently taken up by all three cancer cell lines. In vivo studies were conducted using NOD-SCID mice with a subcutaneous PC-3 human prostate tumor model. These experiments demonstrated that L5 and L6 had a safe profile and consistently led to a significant reduction in tumor volume, confirming their therapeutic potential.
FAQs
Q: How do Peptide-Drug Conjugates (PDCs) compare to Antibody-Drug Conjugates (ADCs) and other targeted therapies?
A: PDCs offer several key advantages over larger modalities like ADCs. The smaller molecular weight of peptides allows for superior tissue and tumor penetration and faster clearance, which can reduce systemic toxicity. Furthermore, the modular and versatile nature of PDCs provides greater flexibility in targeting a wider array of receptors and carrying diverse payloads.
Q: Can a single PDC platform be used for multiple drug classes?
A: Yes, PDCs are highly versatile. The design can be customized to accommodate various drug classes, including small-molecule cytotoxics, immunomodulators, and nucleic acid-based therapeutics. The choice of peptide, linker, and conjugation strategy is precisely tailored to the specific physicochemical properties of your therapeutic agent to ensure optimal stability and performance.
Q: What is the typical project timeline for developing a custom PDC?
A: A standard project duration is contingent upon design intricacy and validation breadth. Core stages typically encompass peptide/linker conception, synthetic chemistry, bioconjugation, and exhaustive in vitro / in vivo validation. We collaborate intensively with partners to define a granular project roadmap and schedule.
Q: How is the stability and on-target drug release of PDCs ensured?
A: We engineer PDCs with advanced linker chemistry, such as pH-sensitive or enzyme-cleavable linkers, that remain stable in circulation but are selectively cleaved by specific conditions at the disease site. This ensures the drug is released precisely where it is needed, maximizing therapeutic efficacy and minimizing premature payload release and off-target side effects.
Q: How can Creative Biolabs' services be tailored to a project with unique therapeutic requirements?
A: Our services are highly flexible and fully customizable. We specialize in bespoke PDC design, which includes tailoring peptide sequences to target a specific receptor, optimizing the drug-to-peptide ratio for the desired efficacy and safety profile, and fine-tuning conjugation chemistry to meet the unique specifications of your therapeutic goals.
At Creative Biolabs, we are committed to helping you harness the full potential of Peptide-Drug Conjugates to create more effective and safer therapeutics. Our comprehensive range of services, from custom synthesis to pre-clinical validation, is designed to support your project at every stage.
Connect with our experts for project-specific consultation and detailed insights.
References
- Jadhav, Krishna et al. "Peptide-Drug Conjugates as Next-Generation Therapeutics: Exploring the Potential and Clinical Progress." Bioengineering (Basel, Switzerland) vol. 12,5 481. 30 Apr. 2025, DOI:10.3390/bioengineering12050481.
- Hoppenz, Paul et al. "Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies." Frontiers in chemistry vol. 8 571. 7 Jul. 2020, DOI:10.3389/fchem.2020.00571.
- Gomena, Jacopo et al. "Targeting the Gastrin-Releasing Peptide Receptor (GRP-R) in Cancer Therapy: Development of Bombesin-Based Peptide-Drug Conjugates." International journal of molecular sciences vol. 24,4 3400. 8 Feb. 2023, DOI:10.3390/ijms24043400.
- Distributed under Open Access license CC BY 4.0, without modification.



