A comprehensive catalog of pre-formulated delivery systems (liposomes, exosomes, LNPs, polymeric nanoparticles) and a selection of validated targeting modules (aptamers, peptides, functionalized lipids, targeted polymers), ready for your research and development needs.
Aptamer-Drug Conjugate (ApDC) Development Service
Are you currently facing the challenges of off-target toxicity, drug resistance, and low therapeutic efficacy in your projects? Creative Biolabs' comprehensive Aptamer-Drug Conjugate (ApDC) Services help you overcome these hurdles and develop highly specific and potent targeted therapies. Through advanced aptamer screening, innovative linker chemistry, and robust conjugation platforms, we empower you to achieve superior therapeutic outcomes and accelerate your drug development timeline.
Aptamer-Drug Conjugates (ApDC)
Aptamer-Drug Conjugates (ApDCs) are a class of targeted therapeutics designed to selectively deliver a potent drug to a specific cell type, primarily for applications in cancer therapy. ApDCs comprise three fundamental elements: an aptamer targeting moiety, a molecular linker, and a biologically active therapeutic compound. This modular structure allows for a high degree of customization and is a key advantage over traditional small-molecule drugs, which distribute non-selectively throughout the body, leading to systemic toxicity and adverse side effects. The aptamer's ability to fold into a specific three-dimensional shape allows it to bind with high affinity to its target, often a protein overexpressed on the surface of tumor cells. This specific binding facilitates the internalization of the entire conjugate, where the payload can be released to exert its therapeutic effect.
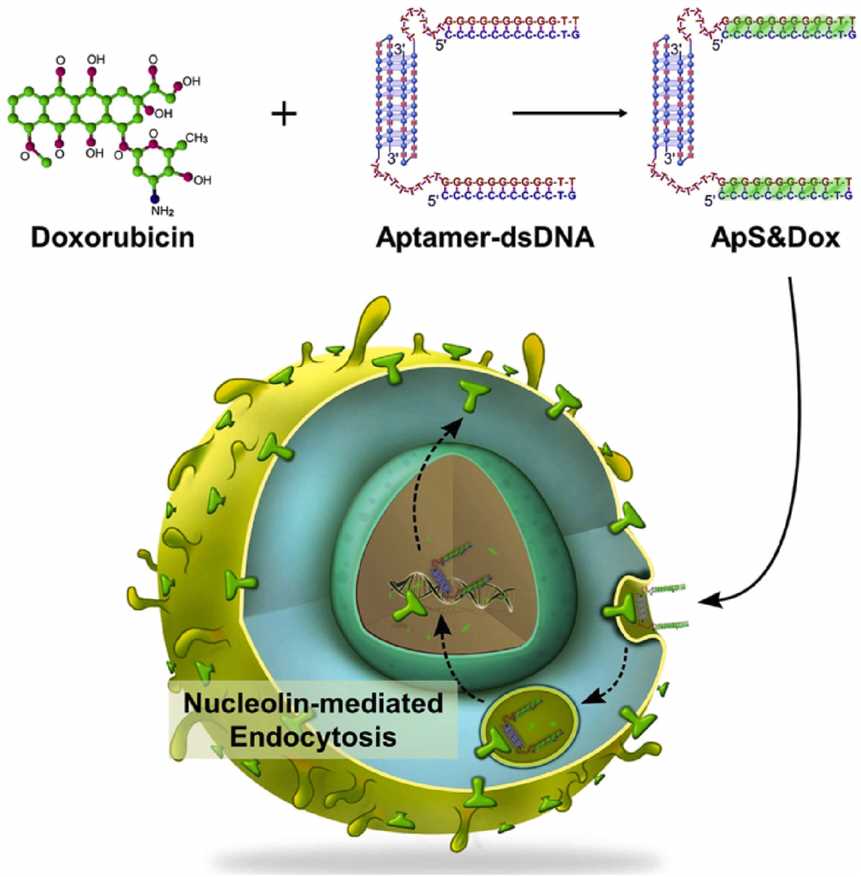 Fig.1 Targeted administration of oncological doxorubicin via functionalized aptamer to overcome MDR mechanisms.1,4
Fig.1 Targeted administration of oncological doxorubicin via functionalized aptamer to overcome MDR mechanisms.1,4
An Aptamer-Drug Conjugate (ApDC) is a targeted therapeutic molecule composed of several key parts, each with a specific function.
The active therapeutic agent, often referred to as the "warhead," is a highly potent compound designed to induce programmed cell death, or apoptosis, particularly in cancer cells. These drugs are extremely toxic on their own, which is why they are conjugated to a targeting agent to ensure they are delivered specifically to the intended cells and minimize harm to healthy tissues. Examples include tubulin polymerization inhibitors, which disrupt the cell's cytoskeleton, and DNA-damaging agents, which prevent cell replication.
Aptamer-Drug Conjugates Methods
ApDCs can be engineered to carry various types of therapeutic payloads, offering versatile solutions for different diseases. The selection of therapeutic agent and bioconjugation element plays a pivotal role in determining conjugate integrity, therapeutic effectiveness, and biological safety. We provide solutions for:
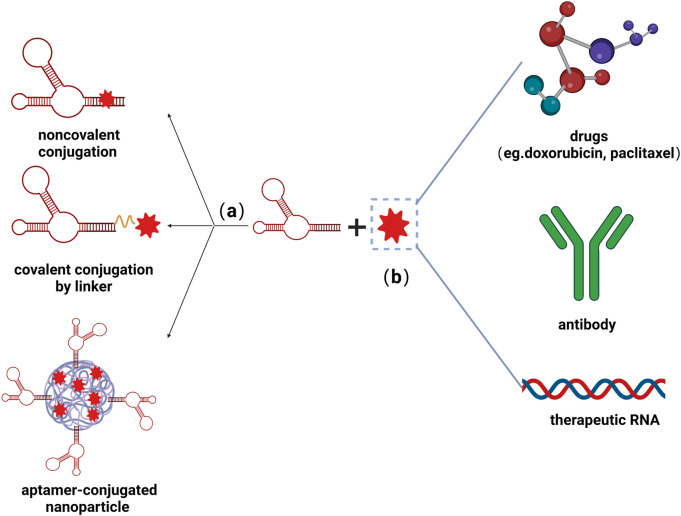 Fig.2 Aptamer-functionalized delivery system.2,4
Fig.2 Aptamer-functionalized delivery system.2,4
- Small-Molecule Drug Conjugates: This involves conjugating a classic chemotherapeutic agent, such as doxorubicin or paclitaxel, to an aptamer. The molecular bridge is engineered to maintain circulatory integrity while enabling selective activation within the target cell's intracellular milieu.
- Nucleic Acid-Drug Conjugates: Aptamers can be used to deliver therapeutic nucleic acids, such as small interfering RNA (siRNA), which can silence specific genes involved in disease progression. This methodology enables precise spatial-temporal regulation of genetic modulation pathways.
- Nanoparticle-Based ApDCs: The aptamer can be conjugated to the surface of a nanoparticle (e.g., liposomes or polymeric nanoparticles) that encapsulates the therapeutic agent. This strategy not only increases the drug payload per conjugate but also provides enhanced stability and a mechanism for controlled, sustained release.
- Radioisotope Conjugates: ApDCs can be designed to carry radioisotopes for theranostic applications, combining diagnostic imaging (e.g., PET, SPECT) and targeted radiotherapy to monitor and treat disease simultaneously.
- Protein/Peptide Conjugates: This involves conjugating an aptamer to a therapeutic protein or peptide, such as a toxin, enzyme, or growth factor, to ensure its targeted delivery and improve its therapeutic index.
Contact Us About Bioconjugation Services
Application
The precision and versatility of ApDCs make them a powerful tool with diverse therapeutic applications, particularly in areas where targeted delivery is critical.
- Targeted Cancer Therapy: This is the most prominent application of ApDCs. By targeting tumor-specific markers, ApDCs can deliver chemotherapeutics directly to cancer cells, reducing the devastating side effects on healthy tissues. This selective targeting can improve the therapeutic index of drugs and help overcome drug resistance.
- Immunomodulation: ApDCs can be designed to deliver payloads that modulate the immune system, such as checkpoint inhibitors or cytokines, directly to immune cells in the tumor microenvironment. This can enhance the anti-tumor immune response while minimizing systemic immune-related adverse events.
- Gene Therapy: Aptamer-mediated delivery of nucleic acid therapeutics provides a non-viral approach for gene therapy, enabling targeted gene silencing or editing with reduced off-target effects and immunogenicity compared to other delivery methods.
What We Can Offer?
Creative Biolabs commands an unparalleled pioneering stance in directed therapeutic delivery progress. Our team of expert biologists, chemists, and engineers brings over two decades of collective experience in developing sophisticated solutions.
Pre-Validated Modules and Delivery Systems
Custom-Engineered ApDC Development
Our wholly customized service creates tailored ApDC constructs from inception through verification, exactly aligning with your initiative's distinctive parameters.
Expertise in Conjugation Chemistry
We possess deep expertise in conjugating selected aptamers to various therapeutic payloads and delivery platforms (nanoparticles, liposomes, etc.).
Comprehensive Pre-Clinical Assessment
We offer in vitro and in vivo testing to rigorously assess targeting efficiency, cellular uptake, biodistribution, and therapeutic efficacy.
Expert Scientific Partnership
Partner with us to leverage our deep scientific knowledge, state-of-the-art facilities, and rigorous quality control for your targeted delivery projects, from experimental design to data analysis.
Workflow

Why Choose Us?
Partnering with Creative Biolabs means choosing a path to accelerated drug development, enhanced therapeutic efficacy, and a significant reduction in off-target effects. Our commitment to innovation and scientific excellence ensures that your therapeutic agents reach their targets with unprecedented precision, unlocking new possibilities for disease treatment.
Unparalleled Scientific Expertise
Our team of highly specialized biologists, chemists, and engineers possesses deep scientific knowledge in targeted drug delivery systems and aptamer development, ensuring your project is in the hands of seasoned experts.
State-of-the-Art Technology Platforms
We leverage cutting-edge platforms for aptamer synthesis, conjugation, and characterization, providing you with the most advanced tools and techniques available.
Fully Tailored Customization
We offer a flexible and collaborative approach, providing customized aptamer design and delivery system optimization to align perfectly with your therapeutic goals and specific targets.
Unwavering Commitment to Quality
Our rigorous quality control protocols and commitment to scientific excellence ensure reliable, reproducible, and high-quality results for your critical projects.
Published Data
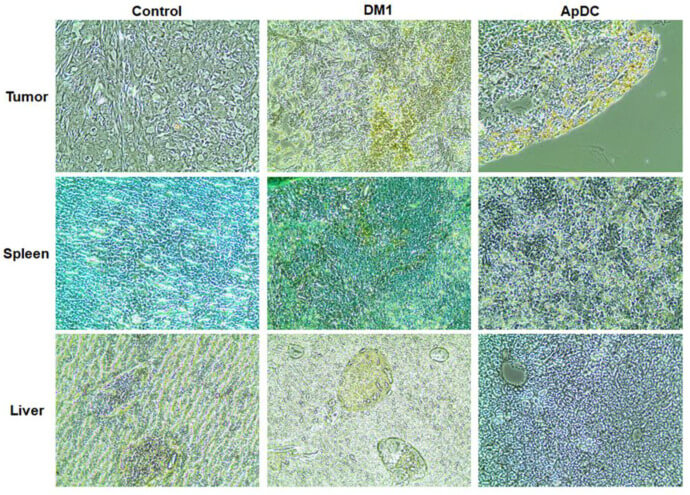 Fig.3 Histopathological alterations following HER2 aptamer-DM1 conjugate introduction.3,4
Fig.3 Histopathological alterations following HER2 aptamer-DM1 conjugate introduction.3,4
The study successfully developed and validated a novel aptamer-drug conjugate (ApDC) for targeted breast cancer therapy. The research utilized a HER2-specific RNA aptamer conjugated to the cytotoxic drug mertansine (DM1). In vitro analysis confirmed the ApDC's high specificity, showing it effectively bound to HER2-positive BT-474 breast cancer cells, with minimal interaction with HER2-negative MDA-MB-231 cells. This specific binding was further visualized through confocal microscopy. The ApDC exhibited potent cytotoxic effects on the targeted BT-474 cells, achieving a dose-dependent reduction in cell viability, while showing significantly lower toxicity to the non-target cells. In vivo efficacy was demonstrated in xenograft mouse models with BT-474 tumors. Mice treated with the ApDC showed a marked reduction in tumor growth compared to control groups. Importantly, this therapeutic effect was achieved without the significant systemic toxicity often associated with traditional chemotherapy, as evidenced by stable body weights and the absence of hepatic damage markers in the treated animals. These findings highlight the potential of aptamer-based delivery systems to enhance therapeutic efficacy and reduce off-target side effects in oncology.
FAQs
Q: What distinct benefits do ApDCs provide compared to conventional ADCs?
A: ApDCs offer distinct advantages due to their small size (6-15 kDa), which enables superior tumor penetration. As chemically synthesized oligonucleotides, they are more cost-effective and have higher batch-to-batch consistency. Furthermore, their low immunogenicity reduces the risk of an adverse immune response.
Q: What strategies are used to enhance aptamer stability and improve pharmacokinetics?
A: Aptamers are susceptible to nuclease degradation and rapid renal clearance. This is typically addressed with chemical modifications such as PEGylation, which increases the aptamer's hydrodynamic radius, extending its half-life. Modifications to the nucleic acid backbone are also employed to further improve stability.
Q: How is high specificity and affinity achieved for aptamers?
A: High specificity and affinity are achieved through the in vitro screening process. This robust method screens vast libraries of nucleic acid sequences to identify those that bind to a specific target with exceptional affinity. Through iterative selection, highly specific aptamers are isolated for therapeutic applications.
Q: Which therapeutic agent categories are compatible with ApDC systems?
A: Aptamers are highly versatile and can be conjugated to a diverse range of therapeutic payloads. Common examples include small-molecule cytotoxins, nucleic acid therapeutics (e.g., siRNA, miRNA), and radioisotopes for theranostic purposes. Aptamers can also be linked to nanoparticles to increase the drug payload. The therapeutic cargo is chosen according to defined biological intervention objectives.
Q: What is the typical development workflow for a custom ApDC?
A: The development workflow for a custom ApDC is a multi-stage process. It begins with aptamer discovery and optimization, followed by linker design and conjugation to the payload. This is succeeded by comprehensive characterization, including data on binding affinity (Kd), stability, and in vitro/in vivo efficacy assessments. The project is designed to deliver validated data at each key milestone.
Creative Biolabs offers a robust and comprehensive suite of ApDC services, from custom aptamer design and synthesis to final conjugate validation. We provide the expertise and technology to transform your therapeutic concepts into reality.
Connect with our experts for project-specific consultation and detailed insights.
References
- Liu, Pingping, et al. "Aptamer-drug conjugates: New probes for imaging and targeted therapy." Biosensors and Bioelectronics: X 10 (2022): 100126, DOI:10.1016/j.biosx.2022.100126.
- Chen, Xinyuan et al. "Aptamer-based applications for cardiovascular disease." Frontiers in bioengineering and biotechnology vol. 10 1002285. 13 Oct. 2022, DOI:10.3389/fbioe.2022.1002285.
- Jeong, Hwa Yeon et al. "Development of HER2-Specific Aptamer-Drug Conjugate for Breast Cancer Therapy." International journal of molecular sciences vol. 21,24 9764. 21 Dec. 2020, DOI:10.3390/ijms21249764.
- Distributed under Open Access license CC BY 4.0, without modification.



