Cell based Vaccine Production Service
Creative Biolabs is a CRO with years of experience in vaccine development. Over the past ten years, we have not only continuously innovated in technology, but also established multiple comprehensive vaccine development platforms. With the accumulated experience and expanded scale, we have established a series of mature vaccine production facilities with GMP qualifications, and we also have successfully provided customers with quality vaccine production services.
GMP-compliant Cell-based Vaccine Production
Large-scale cultivation of mammalian cells has been widely used in the production of biological products such as human vaccines, veterinary vaccines, and gene therapy drugs. The ideal goal is to achieve high-density culture of cells, high-density expression, and to ensure the quality of products produced on a large scale. Currently commonly used cell culture systems include primary cells, diploid cells, passage cells, stem cells, etc., for example, porcine kidney, monkey kidney, hamster kidney cells, 2BS, KMB-17, MRC-5, WS-38, CHO Cells, BHK-21 cells, and Vero cells. Among which, Vero cell is the most satisfactory choice for its stability and well-documented performance in quality and quantity of viral yield. Furthermore, it has also received FDA approval and is being used throughout the world.
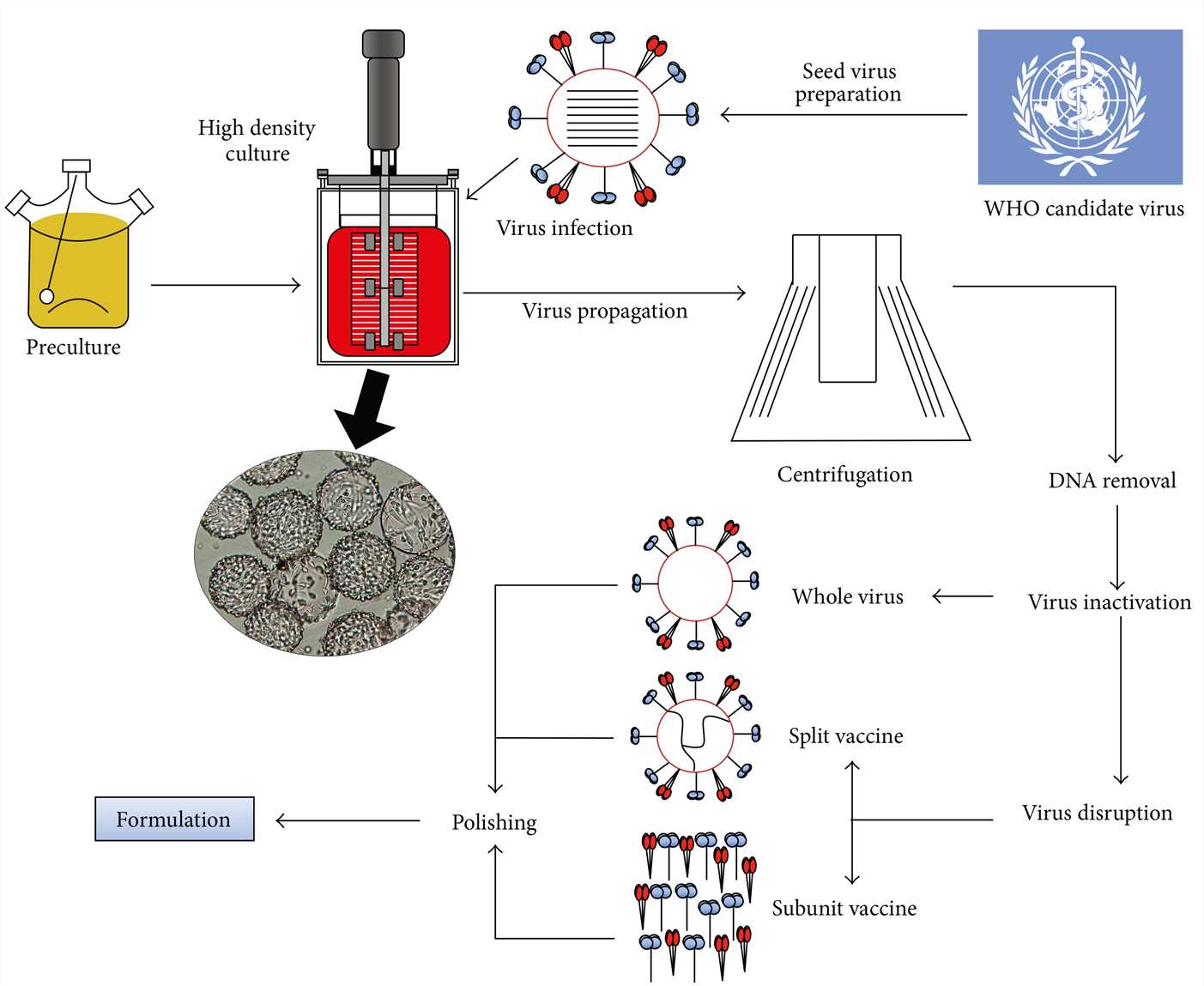 Fig. 1 Schematic flowchart of the upstream and downstream production process of the cell-based vaccine. 1
Fig. 1 Schematic flowchart of the upstream and downstream production process of the cell-based vaccine. 1
The implementation of GMP can maximize the quality of vaccines and significantly increase the competitiveness of companies and their products. Creative Biolabs has extensive experience in vaccine development and is now working with GMP production facility partners. In addition to the Vero cell line, which is commonly used in the industry to produce vaccines, we have also established a variety of other conventional mammalian and insect cell lines that can be selected for production. According to the different scale needs of customers, we can choose flasks, roller bottles and bioreactors for production. Complete downstream purification processes, formulation and filling services further allow you to fully enjoy the high-quality mass production of vaccines.
Features of GMP-compliant Cell-based Vaccine Production Service
- GMP-compliant facilities and environment
- A variety of stable and robust mammalian and insect cell lines
- Customizable production yield
- One-stop vaccine manufacturing capacity from production, purification, formulation to filling
- Ability to produce different viral vaccines
Creative Biolabs is a world-leading CRO/CDMO for vaccine development and production. Harnessed with a world-class research team and highly competitive R&D and production capacity, we have assisted thousands of clients with high-level services in vaccine development. We will continue to fuel the health of humanity with the most professional attitudes and technologies!
Reference
- Milián, Ernest, and Amine A. Kamen. "Current and emerging cell culture manufacturing technologies for influenza vaccines." BioMed research international 2015.1 (2015): 504831. Distributed under Open Access license CC BY 4.0, without modification.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


