Vaccine Combination Immunotherapy
Immunotherapy has become a rising star in the treatment of cancer, but not all patients have a good response to checkpoint blockade, so the combination with other therapies becomes a practical necessity. Creative Biolabs takes full advantages of its own expertise in vaccine development and integrates immunotherapies such as checkpoint blockade to pioneer a vaccine combination immunotherapy, creating a rich pool of strategies to combine vaccine with immunotherapies that will provide a bright future for cancer treatment.
Background
The discovery of immunologic checkpoints and its clinical application make immunotherapy the most sought-after method for treating cancer. Stimulating the body's immune system to target malignant tumor cells to achieve cancer treatment is a long-term attempt by clinicians and scientists. But studies have shown that although patients are able to produce immune responses against tumor antigens, these reactions have no significant clinical effects. The reasons for this result are manifold. Among them, the selection of target antigen is the decisive factor of vaccine immunogenicity. In addition, there is an increasing amount of evidence indicates that the tumor microenvironment interferes with immune cell function through a variety of mechanisms. Therefore, the use of vaccines that employ tumor-specific antigens in combination with immunotherapy that overcomes tumor immune escape is currently a popular and effective strategy for achieving cancer treatment.
Possible Mechanisms for Failure of Cancer Vaccines
Except for the currently widely accepted target antigen selection and the suppression of tumor microenvironment on immune cell function, other factors that have been discovered may also affect the vaccine's effectiveness. In advanced solid tumors with local variation, tissue hypoxia is a common phenomenon. Although there is no direct evidence that hypoxia can affect the anti-tumor function of T cells, a recent study showed that the use of metformin to regulate the hypoxic state of the tumor can not only improve the efficacy of PD-1 blockade but also increase the production of intratumoral effector cytokines. However, studies have also shown that excessive oxygenation in some tissues should be responsible for the dysfunction of T cells against tumor, and similar experiments in mouse models indicate that in some tissues, oxygen can inhibit the anti-tumor effect of T cells. In any case, the oxygen state of the tissue is likely to be a factor in regulating the anti-tumor activity of T cells. The deepening research of this factor is also an avenue for combination therapy of tumor vaccines.
IDO (Indoleamine 2,3-dioxygenase 1) acts as an IFNγ-regulating enzyme to degrade tryptophan and has been found to inhibit the anti-tumor effect of effector T cells or promote the activity of Treg cells. Therefore, IDO inhibitors have also become a trend in anti-tumor drug research. Some animal models and clinical trials are optimistic about the role of IDO inhibitors alone or in combination with vaccines in anti-tumor.
The suppression of tumor cell necrosis on the anti-tumor activity of T cells is an important research result recently revealed. The accumulation of potassium ions released during tumor cell necrosis in anti-tumor T cells inhibits the activation of Akt protein and thus limits the anti-tumor function of T cells. And so, reducing immunosuppression caused by K+ is also a strategy for cancer vaccines to fully exert immunity in the tumor microenvironment.
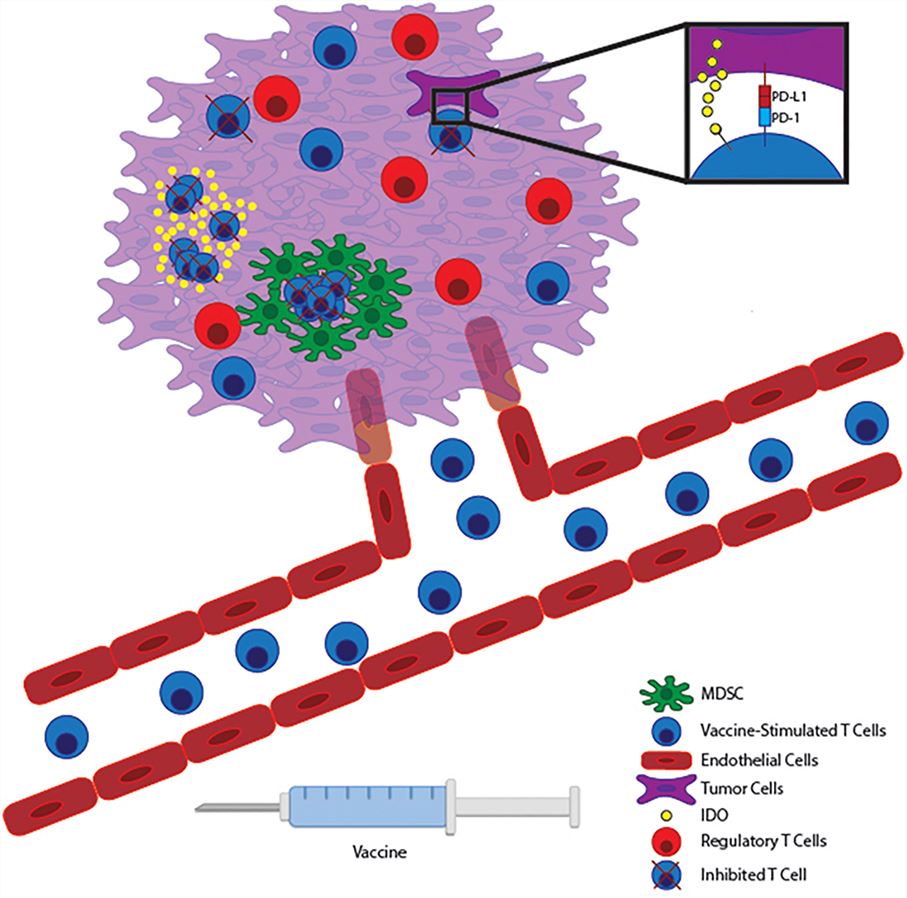 Fig.1 Vaccine-stimulated T cells encounter many immunosuppressive pathways within the tumor microenvironment. 1
Fig.1 Vaccine-stimulated T cells encounter many immunosuppressive pathways within the tumor microenvironment. 1
Combining Vaccines with Immunotherapies
Cancer vaccines include vaccines that typically target shared antigens and vaccines that target neoantigen. The shared antigen is a category of antigens expressed by malignant tumor cells and some non-critical healthy tissues. These antigens are overexpressed in tumor cells but are expressed at low levels on normal cells. Since these antigens are autologous proteins, T cells capable of recognizing such antigens are likely to be deleted during development, thus resulting in a limited number of naïve T cells that can be activated by vaccines targeting such antigens and so to produce insufficient immune responses. Increased immunogenicity of shared antigens has been observed in several clinical trials by altering the anchor residues of the peptide as well as in combination with IL-2. Another type of tumor-associated antigen, neoantigen, has gained increasing attention in the near future. Such personalized neoantigen cancer vaccine uses mutant peptides as antigens which are only present in cancer cells, and because each individual's tumor mutations are unique, mutation-based vaccines are bringing ideas for personalized tumor treatment. Vaccines targeting neoantigen can reduce the non-specific effects of the vaccine on non-tumor tissues as well as autoimmunity, and the initial T cells that recognize such antigens can be prevented from being deleted by the thymus.
Combining Vaccines with Checkpoint Blockade
Vaccine combined with checkpoint blockade is a strategy for treating cancer. The surface of activated T cells is capable of expressing an inhibitory receptor that limits T cell activity. Checkpoint pathway therapies, such as CTLA-4 blockade therapy and PD-1 blockade therapy, are currently approved by the FDA for administration. CTLA-4 is a cytotoxic lymphocyte antigen 4 on the surface of T cells, which binds to the CD28 molecule on the surface of dendritic cells and is capable of limiting the function of T cells. Antibodies against CTLA-4 are effective therapies for the treatment of metastatic melanoma. PD-1, also known as programmed cell death protein 1, binds to PD-L1 and inhibits the activation of anti-tumor T cells by interfering with the signaling pathway of CD28. Both T cells capable of producing interferon and vaccine-induced T cells can enhance the expression of PD-L1, which results in reduction of the vaccine efficacy. Therefore, inhibition of checkpoints while inoculation of tumor vaccines is an effective combination for treating cancer. Some CTLA-4 blockade and PD-1 blockade combined with tumor vaccines show the good effect and feasibility of this therapy in animal and clinical trials. In addition, other T cell costimulatory molecules such as TIGIT, TIM3, LAG3, and BTLA are also being evaluated as components of vaccine combination immunotherapy.
Combining Vaccines with Therapies Targeting Regulatory Immune Cells
Apart from the inhibition of T cell anti-tumor function by checkpoint molecules, some immune cells can also negatively regulate the function of T cells, including MDSCs and Treg (Foxp3+ T regulatory cells). Tregs highly express IL-2 receptor alpha chain and reduce the availability of IL-2 in traditional T cells. In addition, Tregs can regulate adenosine metabolism by secreting anti-inflammatory cytokines and delete effector T cells via a granzyme-mediated mechanism. Recent studies have shown that the expression of CTLA-4 by Tregs is one of the reasons why CTLA-4 blocking antibodies exert the anti-tumor function, which makes it possible for vaccine combination immunotherapy. Therapies targeted at Tregs are currently being evaluated, and existing trials have confirmed that mice that have Tregs removed can delay melanoma growth and improve the efficacy of melanoma vaccines. Myeloid-derived suppressor cells (MDSC) exert immunosuppressive effects on T cells by secreting immunosuppressive molecules and anti-inflammatory cytokines. Meta-analysis shows a correlation between the frequency of MDSC in the blood and the survival rate of cancer patients. Combined with other research results, the therapy targeting MDSCs will likely play a better role in the anti-tumor effect of tumor vaccines.
Our Services in Vaccine Combination Immunotherapy
- Design and prepare various tumor vaccines with good effects
- Design and assessment of different types of immunotherapy
- Production of inhibitors for checkpoint and regulatory immune cells
- Design and evaluation of vaccine combination immunotherapy
The different cancer conditions of different patients make the treatment of cancer complicated, and clinical experience shows that the combination of multiple cancer immunotherapy may be an effective strategy for treating cancer. Creative Biolabs' deep experience and in-depth knowledge in cancer research have made us an expert in the design and development of various immunotherapies and the scientific combination of different therapies for maximum therapeutic efficacy.
Reference
- Grenier, Jeremy M., Stephen T. Yeung, and Kamal M. Khanna. "Combination immunotherapy: taking cancer vaccines to the next level." Frontiers in immunology 9 (2018): 610. Distributed under Open Access license CC BY 4.0, without modification.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


