Exosomes in Targeted Delivery: Engineering and Applications
Exosomes in targeted delivery offer a biology-native way to move RNA, proteins, and small molecules precisely to diseased tissues. Through smart engineering—loading methods, ligand display, and scalable isolation—exosome delivery improves uptake while protecting cargo. At Creative Biolabs, we outline the core engineering choices and real-world applications in oncology and neurology.
What Are Exosomes? Why They Matter for Targeted Delivery?
Exosomes are nanoscale extracellular vesicles (30-150 nm in diameter) naturally secreted by most cell types, from immune cells to stem cells and cancer cells. They are produced by inward budding of endosomal membranes to make intraluminal vesicles (ILVs) within multivesicular bodies (MVBs), which then fuse with the plasma membrane and discharge exosomes into biofluids such as blood, urine, and cerebrospinal fluid. These vesicles are not mere cellular "waste"; they act as natural messengers, shuttling proteins (e.g., tetraspanins CD63/CD9, integrins), nucleic acids (miRNAs, mRNAs), and lipids between cells, enabling intercellular communication in both physiological and pathological processes. Exosomes possess five unique properties that make them excellent candidates for targeted drug delivery.
Biological Compatibility
As they originate from natural cells (e.g., mesenchymal stem cells, autologous cells), exosomes have low immunogenicity and high biocompatibility. This property allows exosomes to avoid strong immune rejection and exhibit reduced cytotoxicity in vivo.
Natural Targeting Ability
Because exosomes possess surface molecules (e.g., integrins αvβ5 for liver, α6β4 for lung) and cell-source tropism (neural stem cell-derived for CNS), they have inherent targeting potential, including organ/tissue tropism and blood-brain barrier (BBB)-crossing capability.
Engineerability
Exosomes are highly engineerable via genetic modification and chemical modification. Therefore, their targeting could be customized to enhance the specificity.
Cargo Protection & Delivery
In the exosome-based delivery, encapsulated cargo (e.g., siRNA, chemotherapeutics) is protected from lysosomal breakdown by fusing with recipient cell membranes or using receptor-mediated transcytosis (RMT). In addition, exosomes can load diverse cargo, including nucleic acids (miRNA, siRNA), small molecules (doxorubicin, curcumin), proteins (enzymes, antibodies), and nanoparticles (SPIONs) via electroporation, incubation, or chemical methods.
Physiological Stability
Exosomes' physiological stability stems from two key traits, both tied to their structural and molecular features, which benefit targeted drug delivery by ensuring cargo reaches target sites. The first one is the long circulation time in the body, which is conferred by the exosomal surface protein CD47. The exosomal surface protein CD47 can interact with signal regulatory protein α (SIRPα) on phagocytes, thus sending a "don't eat me" signal to evade clearance by the reticuloendothelial system (e.g., liver, spleen macrophages).
Exosome Engineering: From Natural Vesicles to Precision Nanocarriers
Loading Strategies
As mentioned before, exosomes can encapsulate diverse payloads, including small molecules, RNA, and proteins (Table 1). Different molecules are loaded onto exosomes by different loading methods for specific purposes.
Table 1 Loading strategies.
| Payload | Loading method | Pros | Watch-outs | Typical use |
|---|---|---|---|---|
| Small molecules (e.g., DOX, PTX) | Passive incubation; membrane permeabilizers | Simple; scalable | Risk of leakage; batch variability | Oncology research |
| si/mi/mRNA | Electroporation; transfection of donor cells | Nucleic-acid ready; endogenous sorting | RNA integrity; dose normalization | Gene-silencing/knock-down studies |
| Proteins/enzymes | Donor-cell expression; chemical conjugation | Functional protein delivery | Activity retention; orientation | Enzyme replacement models |
Surface & Genetic Functionalization
To improve homing and uptake, exosome surfaces can be decorated with various ligands, including peptides, receptor-binding proteins, and antibody fragments (scFvs) via chemical conjugation or genetic display scaffolds (Figure 1). Recent studies show modular EV platforms (e.g., LEAP) that present scFvs such as anti-PD-L1 or anti-CD3 can dock specifically with target receptors and exhibit facilitated receptor-mediated internalization.
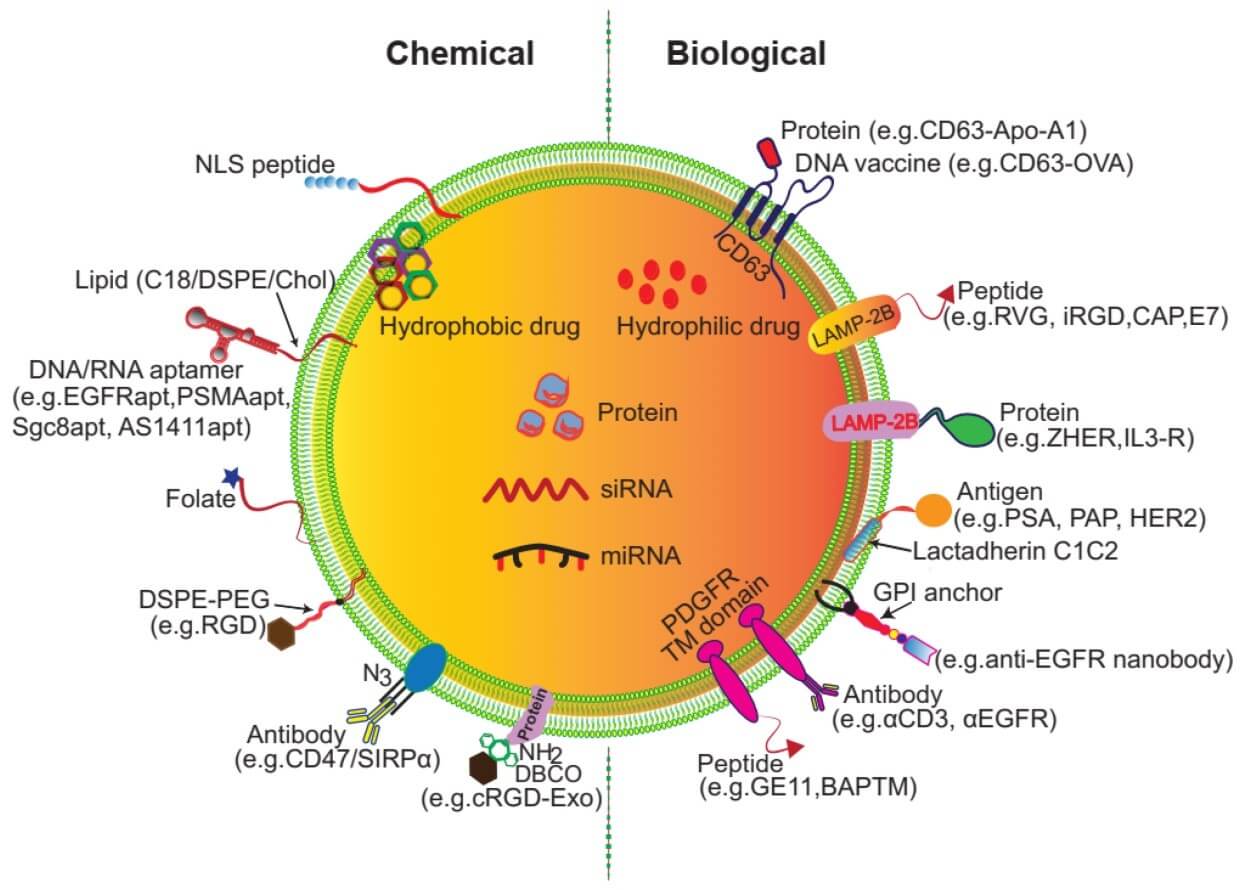 Fig.1
Surface and genetic functionalization of exosomes via genetic manipulation or chemical modification.2
Fig.1
Surface and genetic functionalization of exosomes via genetic manipulation or chemical modification.2
Peptides vs. scFvs
Peptides are compact and easy to scale. However, scFvs can provide higher affinity and specificity when receptor density is low or off-target expression is a concern.
Exosome Mimetics & Hybrid EVs
When yields or batch variability hinder progress, exosome-like mimetics or hybrid liposome-exosome systems can emulate exosomal tropism while easing manufacturing constraints. These delivery systems are helpful in early feasibility stages before locking a GMP path.
Manufacturing & Quality: From Bench to GMP
Isolation & Scale-Up
No single isolation method fits all programs. Integrated TFF→SEC trains are emerging as robust, scalable baselines for therapeutic-grade preparations. Firstly, tangential flow filtration (TFF) concentrates extracellular vesicles (EVs) gently and efficiently; then, size-exclusion chromatography (SEC) is used to remove protein aggregates and small contaminants; finally, ultracentrifugation is applied for protein concentration.
Process flow (typical):
Discovery → Cell source & media optimization → TFF concentration → SEC polishing → Sterile filtration → Fill/finish.
Characterization & Release Testing
A fit-for-purpose analytics panel often includes: particle size (NTA/DLS), morphology (TEM), identity (tetraspanins CD9/CD63/CD81), potency/uptake assays, sterility, and endotoxin. It should be noted that ISEV emphasizes that tetraspanin detection is not exclusive to "exosomes" compared with other extracellular vesicles (EVs); therefore, multi-parametric characterization of exosomes is needed.
Stability & Formulation
Buffer composition, cryo/lyo methods, and storage temperatures influence membrane integrity and cargo retention. Early formulation screens (including lyoprotectants) can de-risk activity loss and aggregation during long-term storage.
Internal links:
• Targeting Module Development Services: https://www.creative-biolabs.com/targeted-delivery/services.htm.
Biodistribution & Biological Barriers
Tumor Targeting Beyond EPR
While EPR (enhanced permeability and retention effect) can facilitate the initial localization of nanoparticles to tumor tissues, the subsequent cellular uptake of therapeutic carriers is governed by multiple key mechanisms, including the density of target receptors on tumor cells, the rate of receptor-mediated endocytosis, and the unique properties of the tumor microenvironment. Ligand-displayed exosomes often outperform passive carriers in this process, particularly when the selected receptor undergoes rapid endocytosis and recycling. This dynamic receptor behavior ensures continuous uptake of exosomes, avoiding prolonged retention on the cell surface and maximizing cargo delivery to the cytoplasm. Moreover, ligand-displayed exosomes can target receptors expressed not only on tumor cells but also on stromal cells (like fibroblasts), disrupting stromal tight junctions through receptor-mediated interactions to enhance deep tumor penetration.
CNS Delivery & BBB Crossing
Many studies have shown that exosomes and engineered EVs can penetrate the blood-brain barrier (BBB), which paves the way for treating glioblastoma and neurodegenerative diseases. Key design strategies include choosing specific ligands, adjusting vesicle surface charge, and optimizing cargo configuration. A major tactic is using receptor-mediated transcytosis (RMT): engineering exosomes to display ligands such as T7 peptide (binds transferrin receptor, TfR) or ApoB (binds low-density lipoprotein receptor, LDLR) on BCECs enables specific BBB crossing (Figure 2). T7-modified exosomes can boost glioblastoma targeting and do not disrupt natural transferrin-TfR binding. ApoB-modified exosomes show greater accumulation in cerebral vessels and longer retention in the brain. By combining exosomes' natural lipid bilayer traits with ligand targeting, BBB penetration, and cargo release into brain cells can be further improved, thus supporting CNS therapeutic applications.
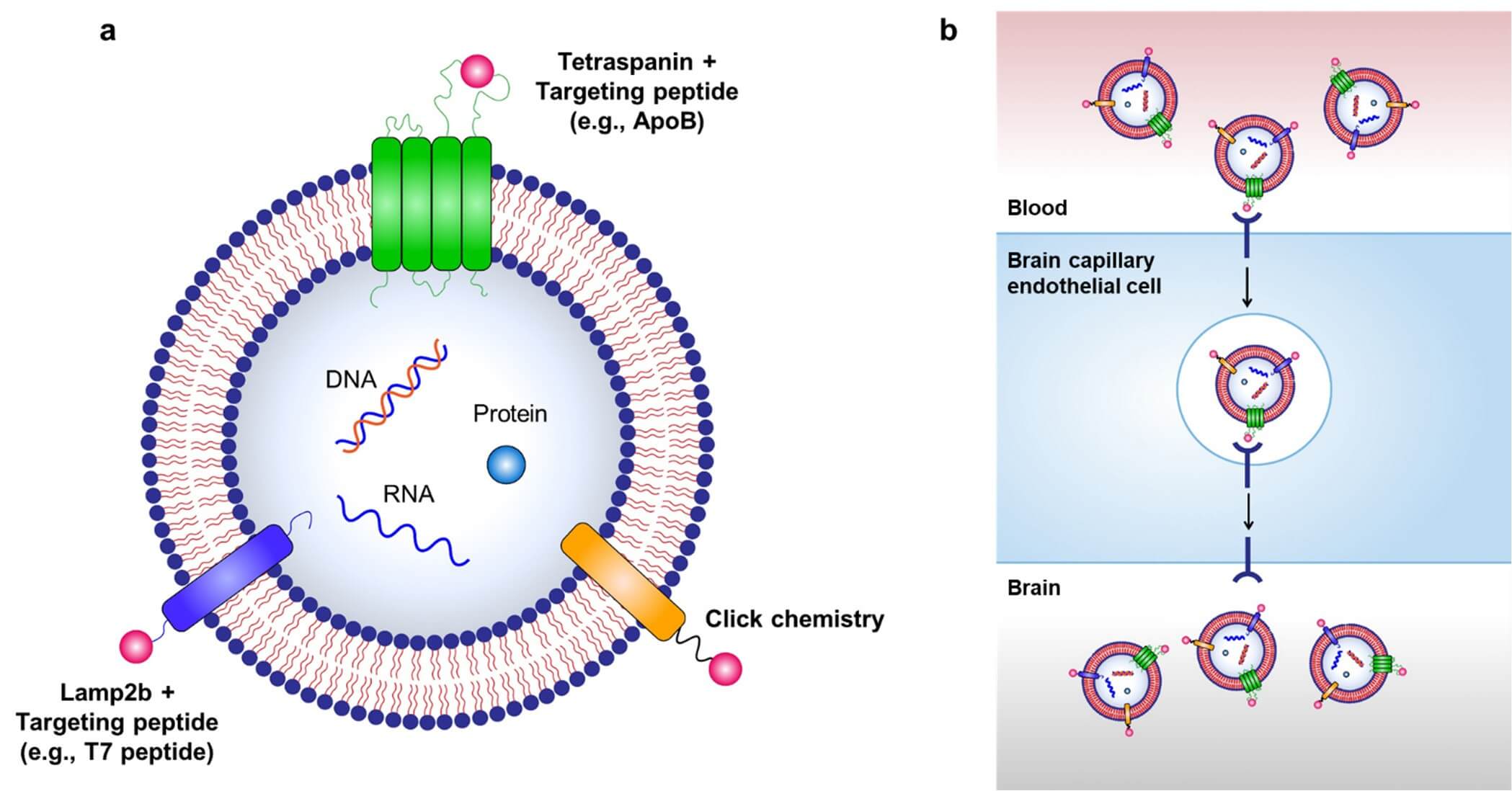 Fig.2
Strategies for targeted delivery of drug-loaded exosomes to the brain.3
Fig.2
Strategies for targeted delivery of drug-loaded exosomes to the brain.3
Immunogenicity & Safety Considerations
In general, the source cells used (e.g., MSCs) as well as the level of purification stringency will influence the cytokine response and biodistribution. At baseline, exosomes derived from MSCs have low immunogenicity, and in clinical studies have been shown to be well tolerated at repeated administration without provoking severe immune rejection or systemic inflammation. In contrast, those prepared from 293T cells might lead to higher antibody levels and more rapid clearance with repeated administration. Design knobs on safety include ligand choice (e.g., transferrin-family targets), vesicle surface charge, and cargo configuration. The characterization of safety profiles requires orthogonal assays, which encompass cytokine array analysis alongside in vivo imaging and organ histopathology examination. It is essential to use orthogonal readouts to provide a comprehensive safety assessment that matches ISEV's primary objective.
Applications & Case-Type Use Scenarios
Oncology
Use cases include chemo-EV combinations for multidrug-resistant tumors, scFv-display EVs for receptor-rich niches, and stromal-targeted payloads in the tumor microenvironment. For multidrug-resistant tumors, macrophage-derived exosomes loaded with paclitaxel (PTX) and engineered with aminoethyl anisamide (AA) (to target lung cancer sigma receptors) can bypass P-glycoprotein drug efflux, thereby boosting PTX accumulation in pulmonary metastases 3.2-fold vs. free PTX to reverse resistance and extend mouse survival. For receptor-rich niches, exosomes displaying anti-EGFR scFv (fused to PDGFR transmembrane domain) can bind EGFR-overexpressing breast cancer cells (e.g., MDA-MB-468), delivering let-7 miRNA to suppress oncogenes and inhibiting tumor growth 2.5 times more effectively than unmodified exosomes. For stromal targeting, exosomes expressing angiopep-2 (targeting LRP1 on cancer-associated fibroblasts) can deliver VEGF siRNA, cutting stromal vascular density by 40% and disrupting the tumor matrix.
Neurology
Exosomes are effective targeted delivery tools for neurological diseases, solving the key problem of crossing the blood-brain barrier (BBB). For Alzheimer's disease, RVG peptide-modified exosomes bind BBB endothelial cells' nicotinic acetylcholine receptors to deliver anti-amyloid β antibodies or miR-29b, reducing amyloid plaques and improving cognitive function in mice. In Parkinson's disease, mesenchymal stem cell-derived exosomes with DJ-1 protein target dopaminergic neurons via CD47, easing neuron loss from α-synuclein aggregation and restoring motor function. For ischemic stroke, angiopep-2-engineered exosomes (targeting LRP1) deliver miR-124, suppressing pro-inflammatory microglia and promoting oligodendrocyte differentiation to reduce infarct size and speed recovery.
How Creative Biolabs Helps
Start here: Targeted Delivery • Targeting Module Development Services
Related Services You May Be Interested in
FAQs
Are exosomes better than liposomes for drug delivery?
They're not "better" in every case, but exosomes offer native proteins and lipids that can enhance cell uptake and biocompatibility; synthetic systems can be simpler to make and customize. Choose based on target biology, manufacturability, and safety.
Can exosomes cross the BBB?
Yes—multiple reviews report BBB traversal and successful brain-targeted delivery strategies with native or engineered EVs.
What limits exosome drug delivery today?
Manufacturing yield, heterogeneity, and standardization. Isolation trains like TFF→SEC and rigorous analytics mitigate many risks.
References
- He, J. et al. "Exosomal targeting and its potential clinical application." Drug Deliv. and Transl. Res. 12, 2385–2402 (2022). https://link.springer.com/10.1007/s13346-021-01087-1.
- Liang, Y., Duan, L., Lu, J. & Xia, J. "Engineering exosomes for targeted drug delivery." Theranostics 11, 3183–3195 (2021). https://www.thno.org/v11p3183.htm. Distributed under Open Access license CC BY 4.0, without modification.
- Choi, H. et al. "Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges." Pharmaceutics 14, 672 (2022). https://www.mdpi.com/1999-4923/14/3/672. Distributed under Open Access license CC BY 4.0, without modification.



