Overview of LNP-based Delivery Strategies for Modern Drug Delivery
What are lipid nanoparticles? How do they work for modern Drug Delivery? In this article, Creative Biolabs provides an overview of the various LNP-based delivery strategies employed in today's biomedical field and introduce the LNP biomedical applications.
Introduction of Lipid Nanoparticles (LNPs)
Definition of LNPs
Lipid nanoparticles (LNPs) are nanoscale lipid carriers with a spherical structure composed mainly of lipids. They are designed to transport nucleic acids, monoclonal antibodies, and small molecules to targeted cells and tissues. Their biocompatibility and adaptability have driven extensive research and application in cancer therapy, gene therapy, and vaccine development. In gene delivery, LNPs shield therapeutic agents from enzymatic degradation or inactivation using ionizable cationic lipids. LNP-based RNA delivery systems have shown enhanced cellular uptake and mRNA expression. Compared to viral vectors, LNPs possess advantages in terms of packaging capacity, ease of modifying structural properties, and scalable manufacturing.
LNP Market in the Biomedical Field
LNPs have recently garnered significant attention in biomedicine. Market reports indicate that the LNP-based gene therapy market surpassed USD 3 billion in 2021 and is growing at an annual rate of approximately 18%. As LNP technology continues to expand into oncology, rare diseases, and precision medicine, the market is poised for accelerated growth in the coming decades.
Types of Lipid Nanoparticles
Based on their general structure, LNPs can be categorized into five subtypes: liposomes, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), nanoemulsions, and lipid polymer hybrid nanoparticles (LPHNPs) (Figure 1). Each of them has characteristic structures and exhibits specific drug-loading properties.
- Liposomes: Liposomes are spherical carriers with an aqueous core surrounded by one or more self-assembled phospholipid membranes. By utilizing the aqueous core and phospholipid membranes, they can be loaded with both hydrophilic and hydrophobic compounds for targeted delivery.
- Solid Lipid Nanoparticles (SLNs): These carriers have a similar general structure to liposomes, yet their core is a solid lipid matrix. The solid core of SLNs allows for enhanced vesicle stability and prolonged release of labile compounds. However, their drug loading capacity is diminished due to the crystalline matrix.
- Nanostructured Lipid Carriers (NLCs): The core of NLCs is composed of a mixture of solid and liquid lipids, distinguishing them from solid lipid nanoparticles. This distinctive core structure not only enables the delivery of lipophilic compounds but also allows for an advanced drug-loading capacity and reduced drug leakage during storage.
- Nanoemulsions: They are usually formed by a dispersed oil phase covered by an outer continuous phase. They can encapsulate either hydrophobic or hydrophilic active compounds as oil-in-water (o/w) or water-in-oil (w/o) droplets. The characteristic nanostructural composition results in enhanced solubility and bioavailability of hydrophobic agents in targeted delivery.
- Lipid-polymer hybrid nanoparticles (LPHNPs): Structurally, lipid-polymer hybrid nanoparticles are composed of a mechanically stable polymeric core and a biocompatible lipid/lipid-PEG shell for enhanced in vivo circulation. The polymeric core can be loaded with various pharmaceuticals, including nucleic acids and peptides. Moreover, targeted drug delivery to specific cells or tissues can be facilitated by incorporating functional groups into the polymer surface.
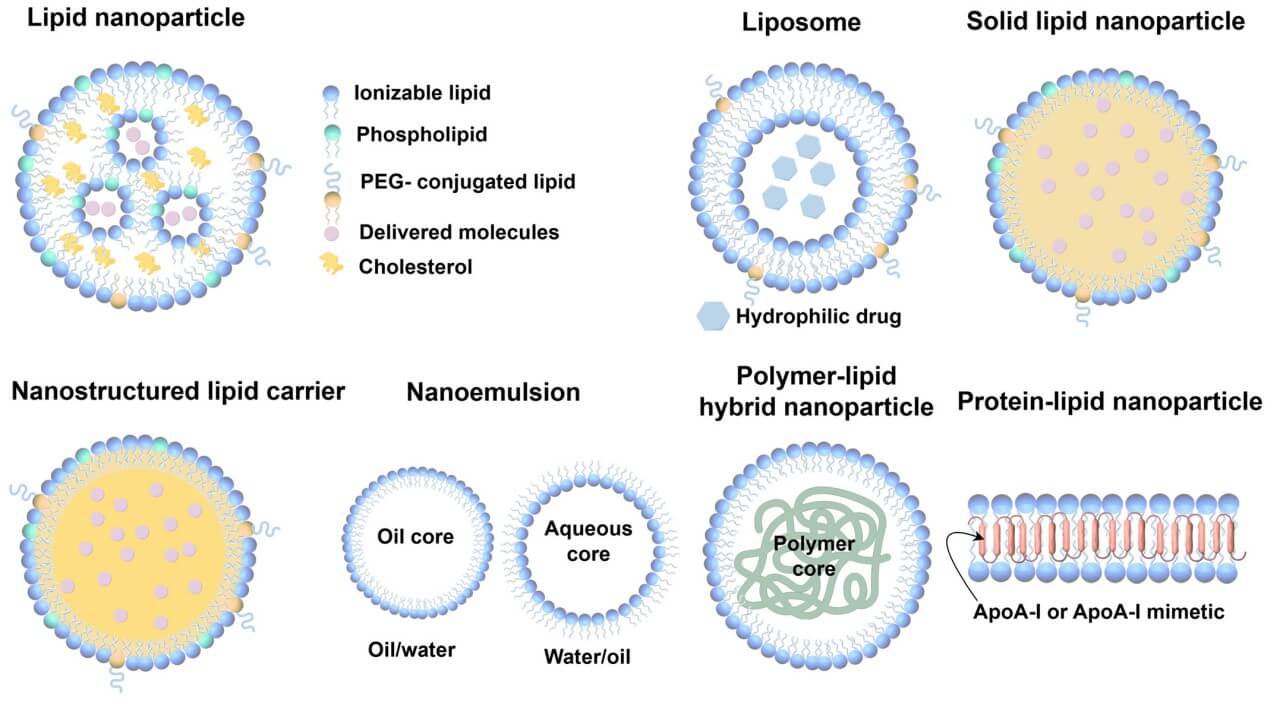 Fig.1 Types of lipid
nanoparticles2.
Fig.1 Types of lipid
nanoparticles2.
Core Components of Lipid Nanoparticles
Classical lipid nanoparticles consist of four core components: phospholipids, cholesterol, PEGylated lipids (PEG-lipids), and cationic/ionizable lipids (Table 1). Each component exerts a specific function and can be modified to improve drug encapsulation efficiency, vesicle stability, or target specificity in drug delivery.
- Phospholipids & Cholesterol: These lipids are responsible for the membrane stability and interactions. The basic structural framework of LNP is primarily constituted by these two components. While phospholipids self-assemble into bilayers or matrices, intercalated cholesterol strengthens membrane stability by regulating lipid packing, reducing membrane fluidity, and preventing particle aggregation.
- PEGylated Lipids (PEG-lipids): PEGylated lipids, such as DSPE-PEG and DSG-PEG, are responsible for the particle stability in vivo. The polyethylene glycol (PEG) chain of PEGylated lipids can extend from the LNP surface, creating steric hindrances that prevent vesicles from being recognized by the immune system. As a result, the in vivo circulation time is extended. However, it is worth noting that PEGylation can reduce cellular uptake.
- Cationic/ionizable Lipids: Cationic or ionizable lipids, such as DOTAP and Dlin-MC3-DMA, are neutrally charged at physiological pH and positively charged in acidic environments, such as the endosomal inside. This property enables the loading of anionic therapeutic drugs (e.g., mRNA and siRNA), improves drug encapsulation efficiency, and facilitates endosomal escape.
Table 1 Core Components of LNPs.
| Component | Proportion | Function in LNPs | Example Molecules |
|---|---|---|---|
| Ionizable lipids | ~50mol% | Encapsulation, endosomal release | DLin-MC3-DMA, ALC-0315 |
| Cholesterol | ~40mol% | Stability, membrane fluidity | Cholesterol derivatives |
| Phospholipids | ~10mol% | Structural support, biocompatibility | DSPC, DOPE |
| PEGylated lipids | ~1.5mol% | Extended circulation, immune evasion | PEG-DMG, PEG-DSPE |
Other factors, such as ligand surface modification, surface charge, particle size, and drug payload percentage, can also influence the stability, cellular uptake, targeting specificity, and biodistribution of LNP vesicles.
LNP Preparation Methods
As lipid nanoparticles have a distinct colloidal structure, the LNP preparation process mainly adheres to the top-down or bottom-up wet chemistry approach. The bottom-up approach is a strategy in which atomic-size materials are nucleated into nanoparticles. There are six methods available for LNP synthesis based on the bottom-up approach. These synthesis methods differ in techniques, conditions, and materials, and produce LNPs with unique biophysical features.
In this method, LNPs were spontaneously formed by mixing miscible organic and aqueous phases through magnetic stirring. The post-formation solvent could be removed via dialysis, ultracentrifugation, or freeze-drying. This method is ideal for encapsulating hydrophobic drugs. Also, it is straightforward, requiring no specialized equipment for synthesis. The common problem with this method is incomplete mixing, which often results in batch-to-batch variation in particle size during scale-up production. In addition, multiple other parameters, including stirring rate and aqueous/organic phase ratio, are found to influence the homogeneity of the LNP synthesized by this method.
This method accommodates various cargo polarities. The hydrophobic drug can be encapsulated via single oil-in-water (o/w) emulsification, which uses low-melting lipids (e.g., stearic acid) and surfactants (e.g., Tween 80) to form crystallized emulsions in cold water. The hydrophilic cargo can be encapsulated via double water-in-oil-in-water (w/o/w) emulsification, containing two-step emulsion formation: formation of a w/o emulsion, followed by dispersion of the w/o emulsion in a hydrophilic emulsifier-containing aqueous phase. The key drawbacks of this method are toxicity from residual solvents and emulsion instability, which can be caused by incomplete solvent removal.
In this method, high-pressure homogenization or ultrasonication is applied to melted lipids and aqueous surfactants to form LNPs with a uniform size. As this method does not use any organic solvents, it is free of solvent-related risks and ideal for encapsulating sensitive cargos, such as siRNA. However, this method's multistep process (variable homogenization time, lipid-surfactant ratio) often leads to significant batch-to-batch variability in particle size, polydispersity, and entrapment efficiency, which hinders industrial scaling.
In this method, LNPs with uniform size are formed by creating a lipid film through rotary evaporation, followed by hydration and extrusion of the thin film through uniform-pore filters. As extrusion parameters (e.g., pressure, membrane pore size, and cycle number) control particle uniformity, and industrial extruders (e.g., LIPEX) handle diverse payloads (e.g., proteins and small molecules), this method is both reproducible and widely used in both laboratory and industrial settings.
This method enables precise and consistent production by carefully controlling the mixing of materials in microchannels. Different microfluidic devices, such as T- or Y-shaped mixers, flow-focusing mixers, and herringbone mixers, offer varying throughput and control over size. To scale up LNP production, devices can be stacked, run in parallel, or combined into a single platform.
This method utilizes fast-velocity streams to produce uniform mRNA-LNP vesicles continuously. It is particularly applied in an industrial setting. However, specialized high-pressure equipment and careful design are required for scale-up production.
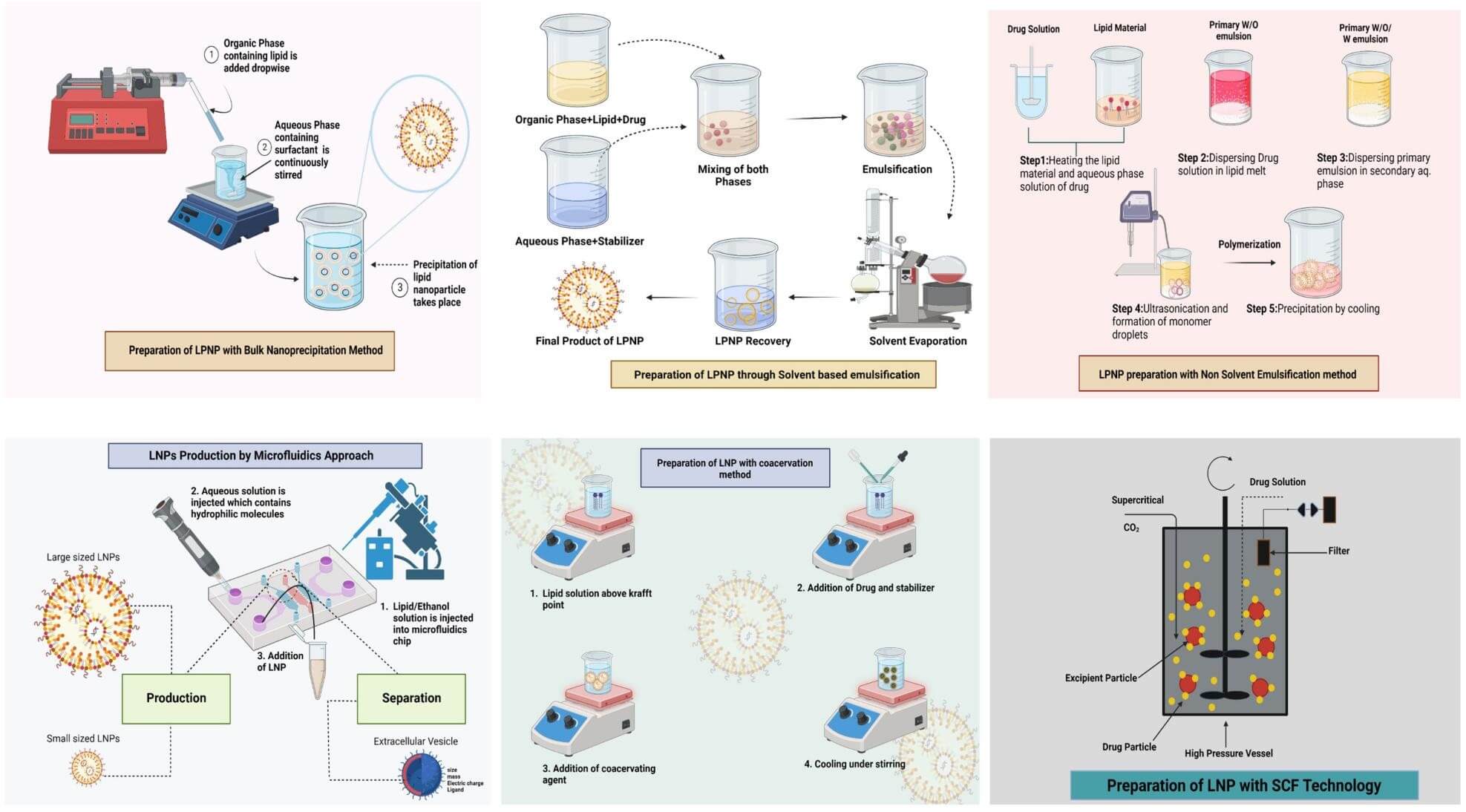 Fig.2
LNP preparation methods1.
Fig.2
LNP preparation methods1.
LNP-Based Targeted Delivery Strategies
LNPs can be targeted to specific cells or tissues using two main strategies: non-ligand targeting and ligand-mediated targeting.
Non-ligand targeting
LNPs can target the liver passively by default, and it can be targeted to specific organs/tissues by modifying their components or charge.
Cationic lipid modifications (e.g., unsaturated tails, constrained adamantane structures) can direct LNPs to the liver (via albumin-associated micropinocytosis) or spleen (via natural T-cell trafficking).
Cholesterol/phospholipid tweaks: esterified/oxidized cholesterol can direct LNPs to liver endothelial/Kupffer cells; phosphatidylserine drives LNP accumulation in secondary lymphoid organs.
PEG-lipid adjustment (alkyl tail length or proportion) enables bone marrow endothelial cell targeting.
Anionic lipids (e.g., CHEMS and 18:1 BMP) can modulate charge for lymph node/spleen targeting.
Helper lipids (phosphatidylserine, constrained phospholipids and anionic phospholipids) can further support organ-specific delivery to lymphoid tissues, liver, or spleen.
Ligand-Mediated Targeting
Ligand-mediated targeting utilizes specific interactions between receptors and ligands to direct LNPs to the target cells/tissues. Two primary methods are applied for attaching ligands to LNPs: post-insertion and direct conjugation.
- Post-insertion conjugation: In this method, ligands are conjugated to preformed LNPs.
Antibodies, such as anti-CD3/CD4 antibodies, can be conjugated to preformed LNPs through thiol–maleimide chemistry, achieving high T-cell transfection.
VCAM-1 antibodies are conjugated to preformed LNPs through SPAAC click chemistry, enabling high brain barrier penetration.
The ASSET system uses bacterial nlpA lipoprotein to anchor scFv, allowing flexible antibody swapping.
- Direct conjugation: In this method, ligands are directly conjugated to LNPs.
Bisphosphonate (BP): They are used to bind and deliver BMP-2 mRNA to hydroxyapatite in the bone by capitalizing on its calcium-ion-chelating characteristics.
Mannose: As mannose receptors are highly expressed on antigen-presenting cells (APCs), LNPs formed by lipids that have mannose conjugation can target these cells specifically.
Dual Ligands: Targeting ligands can be combined (dual ligands) to increase targeting specificity towards certain cell types. For example, the specificity of LNPs to liver sinusoidal endothelial cells (LSECs) can be improved by conjugating mannose and apolipoprotein E (ApoE).
Key Applications of Lipid Nanoparticles
As LNP offers many advantages in terms of safety, encapsulation efficiency, drug efficacy, and scalability in targeted delivery, it has been widely applied in modern biomedical fields, including mRNA vaccines, gene therapy, oncology, and personalized medicine.
mRNA Vaccines
The emergence of the COVID-19 vaccines has proved the pivotal role of LNPs in these mRNA vaccines. In these vaccines, LNPs not only serve as a protective shield for the unstable mRNA but also enhance their cellular uptake, triggering a robust and long-lasting immune response. Beyond COVID-19, the LNP-mRNA platform is being extensively applied to other infectious diseases, such as influenza, HIV, Zika virus, and respiratory syncytial virus (RSV).
Gene Editing
LNPs are versatile and can deliver various types of nucleic acids, such as mRNA, siRNA, and plasmid DNA, to specific tissues and organs. Therefore, LNPs are an advanced alternative to viral vectors for gene therapy. For instance, targeted delivery of the siRNA by LNPs can be applied for the treatment of the genetic diseases, such as transthyretin-mediated amyloidosis. LNPs are also being explored as a potential carrier of gene-editing tools, which could provide a permanent therapeutic solution by correcting genetic mutations within a patient's own cells.
Oncology
LNPs are a promising option for delivering a diverse range of cancer therapeutics. By conjugating tumor-targeting ligands, LNPs can deliver therapeutical drugs more specifically and directly to tumors. They are also being developed for applications in personalized cancer vaccines. By delivering mRNA that encodes for specific neoantigens from the patient's cancer cells by LNP vesicles, the patient's immune system can be trained to specifically recognize and attack their own tumor cells.
Challenges in LNP application
Although LNPs as vesicles have many advantages in safety, encapsulation efficiency, drug delivery efficiency and scalability in targeted delivery, many challenges remain in the LNP application.
Toxicity and Immune Response
Non-biodegradable lipids can accumulate in organs, causing immune responses and a long-term toxicity. In this case, surface modifications (e.g., PEG) and the incorporation of biodegradable linkers can help to minimize the long-term toxicity.
Scalability and Manufacturing Consistency
Although the LNP preparation is well established, it is challenging to maintain the stability, size uniform, encapsulation efficiency and batch-to-batch homogeneity of LNP during the scale-up production. As mentioned before, many parameters can influence these characteristics, such as temperature and stirring rate. Therefore, how these parameters influence the manufacturing consistency during the industrial production should be extensively studied and characterized.
Regulatory Pathways
As the approval of mRNA vaccines has set some precedent, the appropriate regulatory considerations for LNP clinical application regarding safety, biodistribution, off-target effects, and immunogenicity should be determined.
Future Perspectives: The Next Phase of LNP-based Targeted Delivery
Beyond addressing the issues mentioned above, the next phase of LNP-based targeted delivery is expected to link to the advancements in LNP design and application.
In order to deliver larger mRNAs, combinations (e.g. multiple mRNAs or mRNA + siRNA), or gene editing tools by LNP vesicles, the encapsulation strategies and escape efficiencies of LNP are characterized and studied for improvement.
In addition to vaccines, gene therapy, cancer immunotherapy and genetic diseases, LNP vesicles are being explored for applications in the treatment of central nervous system (CNS) diseases and respiratory diseases.
To speed up the LNP designing process, libraries of potential lipids are being screened computationally. AI models can virtually predict key properties (e.g., pKa, delivery efficiency and toxicity), thus accelerating the LNP design process.
Cell-derived membranes and advanced ligand conjugation chemistries are being considered for the engineering of the LNP surface to adjust their biodistribution and cellular uptake.
FAQs
How do lipid nanoparticle delivery systems work?
Lipid nanoparticles (LNPs) exert different functions in different steps of targeted delivery (Figure 3):
- Protection: The LNP's lipid shell is like a physical shield that could protect its fragile cargo, such as mRNA, from degradation by enzymes in the bloodstream. Also, LNPs with a neutral or slightly negative surface charge, can avoid being recognized by the immune system in the bloodstream.
- Cellular Uptake: LNPs can facilitate the endocytosis process via their biomimetic membranes. Moreover, incorporation of targeting ligands can promote the targeting specificity.
- Endosomal Escape: As the endosome becomes more acidic, the LNPs with ionizable lipids will become positively charged, thus disrupting the endosomal wall and escaping into the cell cytoplasm.
- Payload Release: LNPs tend to release its therapeutic payload in the cytoplasm.
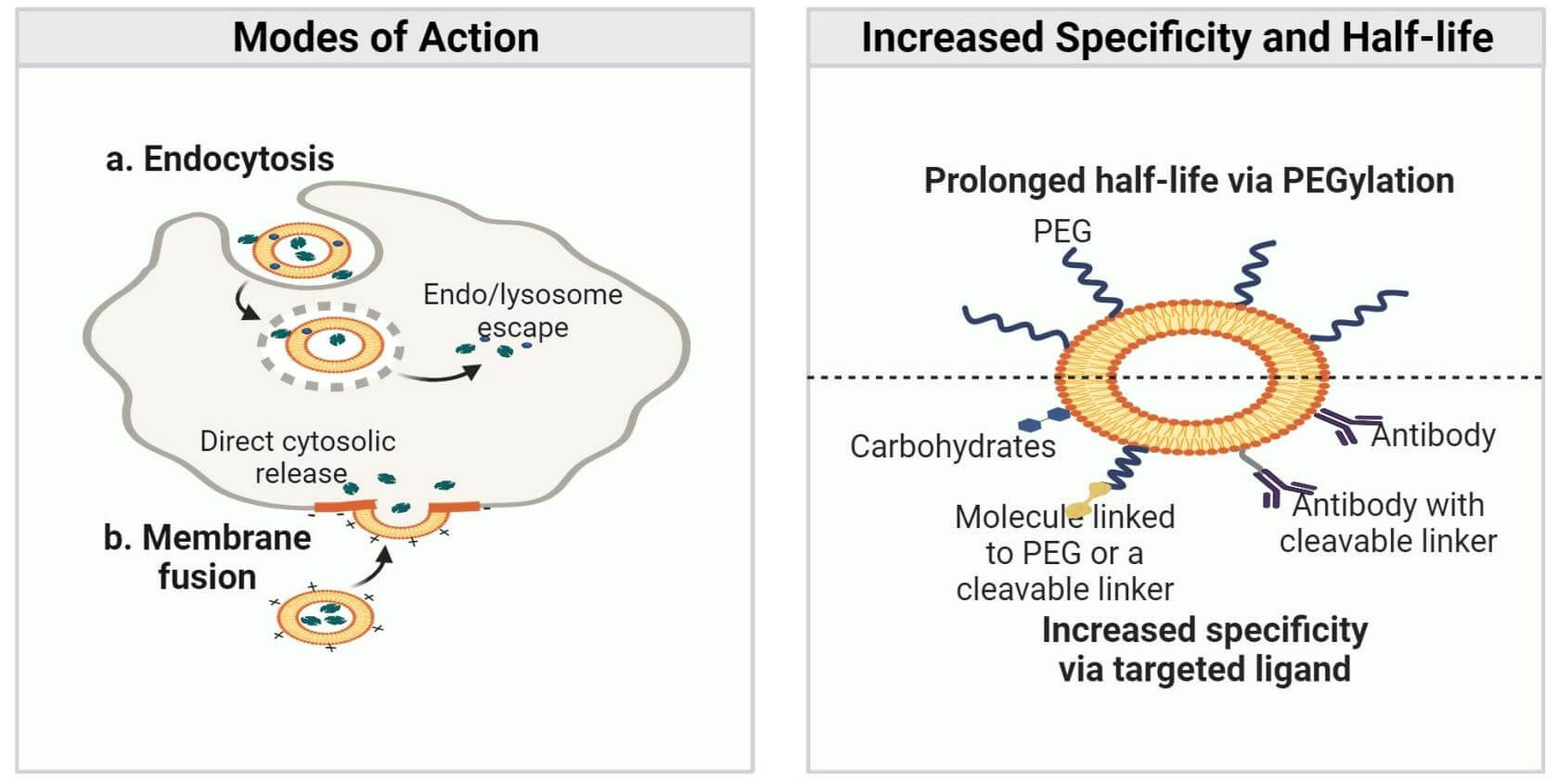 Fig.3 LNP-based
targeted delivery1.
Fig.3 LNP-based
targeted delivery1.
What strategies can improve mRNA LNP delivery?
There are five strategies to improve mRNA LNP delivery:
- Lipid Chemistry: The tail structures and linker chemistry of LNP vesicles are intensively investigated to improve their endosomal escape ability.
- Formulation Optimization: The physical properties of the LNP, including its size, surface charge, and lipid composition ratio, should be precisely tuned to achieve the optimal cellular uptake and minimized toxicity.
- Surface Modifications: PEG modification and incorporation of specific ligands to the LNP surface can enable extended in vivo circulation and targeted delivery to specific cell types or organs.
- Co-delivery: Co-delivery of mRNA vaccine and adjuvants can boost the therapeutic effect.
- Administration Route: The route of administration (e.g., intramuscular, intravenous, or subcutaneous) should be chosen carefully based on the target tissue and the desired therapeutic effect.
Related Products and Services You May Be Interested in
References
- Waheed, I. et al. "Lipid-based nanoparticles as drug delivery carriers for cancer therapy." Front. Oncol. 14, 1296091 (2024). https://www.frontiersin.org/articles/10.3389/fonc.2024.1296091/full. Distributed under Open Access license CC BY 4.0, without modification.
- Zou, Y. et al. "Targeting Neuroinflammation in Central Nervous System Diseases by Oral Delivery of Lipid Nanoparticles." Pharmaceutics 17, 388 (2025). https://www.mdpi.com/1999-4923/17/3/388. Distributed under Open Access license CC BY 4.0, without modification.
- Lee, D. et al. "Strategies for targeted gene delivery using lipid nanoparticles and cell-derived nanovesicles." Nanoscale Adv. 5, 3834–3856 (2023). https://xlink.rsc.org/?DOI=D3NA00198A.
- Shahzad, A. et al. "Innovative lipid nanoparticles: A cutting-edge approach for potential renal cell carcinoma therapeutics." Biomedicine & Pharmacotherapy 180, 117465 (2024). https://linkinghub.elsevier.com/retrieve/pii/S0753332224013519.
Created in September 2025



