Liposomal Delivery Strategies: Design, Loading, Targeting, and Industrial Translation
Introduction to Liposomal Delivery Systems: Definition and Importance
Definition of Liposomal Delivery Systems
Liposomal delivery systems are one of the most mature targeted delivery systems. They can load and deliver various kinds of molecules by leveraging the biophysical properties of liposomes in targeted drug delivery. Structurally, liposomes are spherical carriers with an aqueous core surrounded by a self-assembled phospholipid membrane (Figure 1). Hence, they can be loaded with hydrophilic and hydrophobic molecules with aqueous cores and lipid membranes. In addition, liposomes with a wide range of sizes can be synthesized, further enhancing their loading diversity and capacity. In drug targeted delivery, liposomes with a diameter range of 50-200nm are commonly selected for improved delivery efficiency. Except for being a versatile vessel, liposomes exhibit the following superior clinical properties compared with traditional drug delivery systems:
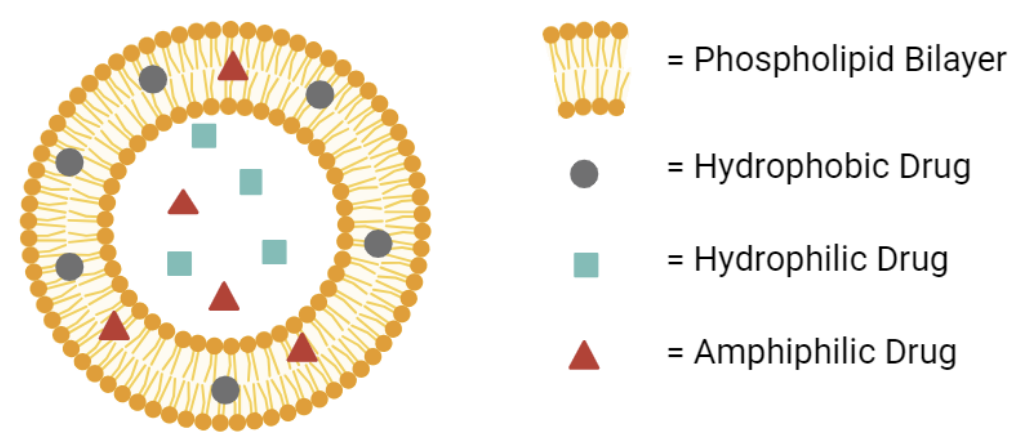 Fig.1 The general structure of liposomes as carriers.2
Fig.1 The general structure of liposomes as carriers.2
Importance of Liposomal Delivery Systems
In 2023, the global liposomal drug delivery market was approximately USD 4.2 billion, and is expected to expand with a compound annual growth rate (CAGR) of 8.2%. The economic growth is believed to be mainly attributed to the increasing application of liposomes in the pharmaceutical field. Since 1995, more than 14 liposomal drug products with indications spanning the fields of oncology, infectious disease, and regenerative medicine, were approved by the Food and Drug Adminitration. For example, doxorubicin liposome for oncology , and liposomal amphotericin B for antifungal treatment , both show better drug efficacy and reduced toxic side effects compared with their counterpart free drugs. Recently, liposomes have also been applied in the cosmetic and nutrition fields for delivery efficiency enhancement. Therefore, this article will review the liposomal delivery strategies regarding the design, loading, targeting and industrial translation.
Liposome Composition and Design: Key Components and Their Roles
As mentioned before, the biophysical properties of liposomes are dictated by the structure and components of liposomes. Commonly, liposomes are composed of 4 types of lipid components:
- Phospholipids: Since saturated lipids increase the rigidity and unsaturated lipids contribute to the fluidity of liposomal membranes, the circulation stability and release profile of liposomal delivery systems can be regulated by changing the saturation level of the phospholipid bilayers.
- Cholesterol: The stability of the liposomal carrier can be strengthened by intercalation of cholesterol into the bilayers.
- PEGylation: Polyethylene glycol (PEG) chains added to phospholipids can function as a "stealth" to the membrane, protecting loaded liposomes from mononuclear phagocytosis. In the liposomal doxorubicin formulation, it is a key feature to prolong the systemic circulation of therapeutic drugs.
- Charged Lipids: In liposome preparation, positively charged lipids are added to bind nucleic acids electrostatically, and negatively charged lipids are used to promote fusion with certain cell membranes.
- Surface Ligands: In addition to the basic lipid components of liposomes mentioned above, ligands, such as antibodies, peptides, or aptamers, can be conjugated to the liposomal membranes for targeted delivery to cancer cells, immune cells, or inflamed tissues.
Common Techniques for Liposome Preparation
There are five classic methods for liposome preparation. Each of them has trade-offs with respective to encapulation efficiency (EE), particle size and polydispersity.
- Solvent Injection Technique: In this method, lipids were mixed with ethanol (or other miscible organic solvent) and then directly injected into an aqueous solution. The rapid dilution of the solvent will lead to spontaneous lipid self-assembly into vesicles. The liposome produced lacks size homogeneity. Therefore, although this method is simple, it cannot be used for industrial liposomal production.
- Reverse Phase Evaporation Method: In this method, the organic solvent is first emulsified with an aqueous solution containing the drug to be encapsulated. Then, lipids are dissolved in the mixture, followed by the removal of the organic solvent under reduced pressure. This liposome preparation exhibits high encapsulation efficiencies of hydrophilic compounds. Although it is very versatile, it is technically difficult to remove all the solvent. Therefore, this method cannot be applied to industrial production.
- Extrusion: Multilamellar vesicles can be extruded through membranes with specific pore sizes to produce unilamellar vesicles. Liposomes produced by this method exhibit improved homogeneity. Therefore, it is commonly used in laboratory and preclinical stages.
- Microfluidics: In this method, microfluidic devices, which can precisely control the mixing of the lipid and aqueous phases, are applied for clinical industrial production of liposomal vesicles.
- Freeze–Thaw Cycling: This method involves repeated freezing and thawing of liposome preparations to transiently incorporate macromolecules into disrupted bilayers. It is often used for laboratory-scale formulations. Also, it should be noted that repeated cycling could damage the stability of lipids.
Table 1 Liposome preparation strategies.
| Method | Typical size | Scalability | Notes |
|---|---|---|---|
| Solvent Injection Technique | 50–150 nm | Moderate | Simple, compatible with ethanol |
| Reverse Phase Evaporation | 100–200 nm | Moderate | Requires organic solvents |
| Extrusion | 100–400 nm | Moderate | Easy, low EE for hydrophilic drugs |
| Microfluidics | 50–120 nm | Excellent | Emerging industrial standard |
| Freeze–Thaw Cycling | 100–400 nm | low | reduced stability |
Drug (or Payload) Loading Strategies in Liposomes
Drugs are encapsulated in proportion to their aqueous or lipid solubility during liposomal formation. Hydrophilic molecules go to the aqueous core, hydrophobic drugs to the bilayer. Simplicity comes with low EE (10–30%) and uncontrolled release.
Pre-formed liposomes with ion/pH gradients actively attract drugs inside. Achieves >90% EE, high dose delivery with minimal leakage. Doxil® uses an ammonium sulfate gradient for doxorubicin.
Co-loading chemotherapeutics with adjuvants, peptides, or siRNA for synergistic effects. Controlled loading ratios and dual-release profiles are under active investigation.
Table 2 Drug loading strategies in liposomal delivery
| Loading Method | Encapsulation Efficiency | Advantages | Limitations | Example Application |
|---|---|---|---|---|
| Passive Loading | 10–30% (varies) | Simple, rapid | Low efficiency, unstable release | Hydrophilic small drugs |
| Active Loading | > 90% | High efficiency, controlled release | Requires ion gradients, additional steps | Doxorubicin (Doxil®) |
| Active Loading | n/a | synergistic effects | immature technology | Chemo + siRNA liposomes |
Targeted Delivery Strategies Using Liposomes
According to different types of target cells or tissues, targeted delivery strategies using liposomes are applied.
- Tumor Targeting: Liposomes can be passively targeted to tumors through the EPR effect. The leaky vasculature in tumors allows liposomes to accumulate more readily in tumor cells than in normal tissues. Moreover, active targeting through the conjugation of antibodies or peptides can be used to further enhance tumor selectivity and accumulation in tumor tissues.
- Immune Cell Targeting in Vaccine Delivery: In vaccine development, delivery of antigens to antigen-presenting cells (APCs), such as dendritic cells and macrophages, plays a key role in triggering an immunological response. Liposomal adjuvants were developed to deliver antigens more effectively to these immune cells. Cationic liposomes have been proven as a potent stimulator of APCs in vaccine development against cancer and infectious disease vaccines.
- Ligand-Mediated Targeting: Targeting liposomes with ligands can promote the endocytosis of liposomes through receptor–ligand interaction (Figure 2). For example, folate-targeted liposomes can be applied to target folate receptor–positive cancer cells. Antibody-conjugated liposomes could provide subtype specificity of targeted liposomes.
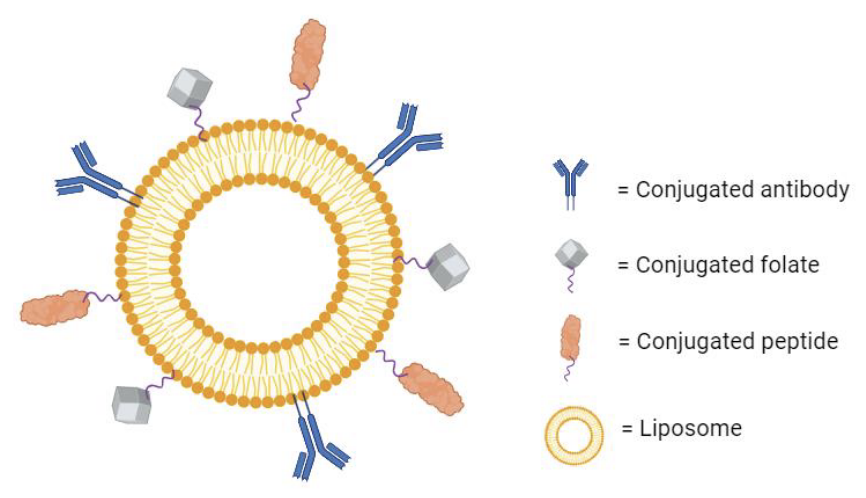 Fig.2 Ligand-mediated targeting in liposomal drug delivery.2
Fig.2 Ligand-mediated targeting in liposomal drug delivery.2
Strategies to Improve Intratumoral Distribution
While liposomes tend to accumulate in tumors, their intratumoral distribution is limited by the dense extracellular matrix (ECM) and high interstitial pressure. Strategies to improve penetration and distribution of liposome-based drug delivery include:
The ECM proteins can be degraded by enzymes such as collagenase or hyaluronidase, and abnormal tumor vasculature can be normalized with anti-angiogenic therapy. Therefore, the use of enzymes and anti-angiogenic therapy is recommended to improve tumor penetration.
Thermosensitive or photosensitive liposomes can be applied to control the drug release for enhanced drug efficacy. For example, lyso-thermosensitive liposomes (LTSLs) can be triggered to release the therapeutic agents near the targeted tumor when the tumor microenvironment is heated to 42 °C.
Short peptides, such as iRGD, have been proven to stabilize the liposomal vesicles and enhance the liposomal penetration into the tumor parenchyma by conjugating to the liposomes.
Direct intratumoral injection can be applied to circumvent vascular barriers and ensure high local drug concentration in the tumor microenvironments.
Emerging Applications of Liposomes
Besides what was mentioned in the introduction, liposomes possess a high potential to be applied in many other biomedical fields.
- Anti-bacterial biofilms: Bacterial biofilms not only physically defend against antibiotic drugs but also render antibiotic resistance via alteration of the microenvironments. In liposome-based drug delivery, liposomes can promote the antibiotic penetration into the bacteria by disrupting the biofilms and prolonging the release of encapsulated antibiotics.
- Drug delivery to the Central Nervous System (CNS): The blood–brain barrier (BBB) is a highly selective physiological barrier for drug delivery to the CNS, as it restricts the passage of most therapeutic agents from the systemic circulation. Fortunately, liposomes decorated with specific ligands were found to enhance BBB penetration into the CNS. Currently, liposomes conjugated with transferrin or ligands of the insulin receptor are being developed to cross the BBB for glioblastoma and other neurodegenerative diseases.
- Delivery of nucleic acids and mRNA: Nucleic acids, including mRNA, small interfering RNA (siRNA), and CRISPR-Cas9 gene-editing tools, are a transformative class of therapeutics with high potential. However, their clinical translation is hindered as these molecules are intrinsically labile in the body. In this scenario, liposomes are a promising delivery technology for RNA therapies as they can protect RNA from degradation and promote its penetration into the target cells. A good example of this is the success of the COVID-19 mRNA vaccines, which cemented lipid nanoparticles.
Emerging Applications of Liposomes
Despite the advantages, some issues related to liposomal application to drug delivery remain challenging for clinical translation:
Drug leakage during storage could occur as lipids tend to undergo oxidation and hydrolysis.To address this issue, PEGylation and lyophilization can be used to enhance the shelf-life and stability of the liposomal vesicles.
Traditional industrial-scale liposome production struggles with reproducibility. However, microfluidics and continuous-flow manufacturing are being developed to enable highly reproducible batch production.
In-depth characterization of particle size, zeta potential, and encapsulation efficiency is required to investigate its potential clinical translation. Understanding the regulatory requirements, Regulatory agencies such as the FDA and EMA have recently released new guidance documents to facilitate approval processes.
Although liposomes posess low immunogenicity, repeated doses of drugs conjugated with liposomes could activate the complement system, thus causing the complement activation-related pseudoallergy (CARPA). The immunogenicity can be redueced by adjusting the lipid composition or addition of PEG.
Emerging Applications of Liposomes
The next phase of liposomal delivery is linked to advancements in precision medicine and next-generation biologics. In oncology, liposomes are expected to be applied together with immune checkpoint inhibitors and adoptive cell therapies. For infectious diseases, therapeutic agents conjugated with liposomes are explored as both antimicrobial carriers and immune adjuvants. The incorporation of artificial intelligence in lipid formulation design is expected to enable the optimization of lipid choice, encapsulation techniques, and release kinetics. Similarly, real-time imaging technologies might allow tracking of liposomal biodistribution in patients and enable dynamic dosing adjustments in the future.
FAQs
What is liposomal delivery?
Liposomal delivery is a type of drug delivery system that uses liposomes——microscopic vesicles——to encapsulate and transport drugs, nutrients, or other bioactive compounds. Compared to conventional drug administration, liposomal drug delivery offers several benefits such as increased bioavailability, reduced toxic side effects, and controlled drug release.
How are liposomes used in drug delivery?
Since liposomes are composed of an aqueous core enclosed by a phospholipid membrane, liposomes can deliver hydrophilic drugs by encapsulating them in the aqueous core and hydrophobic drugs by embedding them in the membrane.
Conclusion
Liposomal delivery systems have emerged as a transformative and indispensable platform in modern nanomedicine, with decades of research and clinical translation validating their unique value in addressing unmet medical needs. From their fundamental design, which leverages phospholipid bilayers to encapsulate both hydrophilic and hydrophobic payloads, to the refinement of advanced strategies like active drug loading, ligand-mediated targeting, and stimuli-responsive release, liposomes have continuously evolved to overcome the limitations of traditional drug delivery systems. The liposomal application is expected to extend to broader clinical adoption in the future.
Related Products and Services You May Be Interested in
Liposome Based Delivery System
References
- Akbarzadeh, Abolfazl, et al. "Liposome: classification, preparation, and applications." Nanoscale research letters 8.1 (2013): 102. https://doi.org/10.1186/1556-276X-8-102. Distributed under Open Access license CC BY 4.0, without modification.
- Gatto, Matthew S., McNeely P. Johnson, and Wided Najahi-Missaoui. "Targeted liposomal drug delivery: Overview of the current applications and challenges." Life 14.6 (2024): 672. https://doi.org/10.3390/life14060672. Distributed under Open Access license CC BY 4.0, without modification.
Created in September 2025



