AAV Vector Design Service for Gene Replacement
Introduction
Creative Biolabs' custom AAV Design for Gene Replacement service addresses gene therapy challenges like targeted delivery, immune response mitigation, and durable transgene expression via advanced capsid engineering and optimized transgene cassettes. We offer an end-to-end solution, guiding projects from concept to clinically-ready vectors, with meticulously engineered AAV, detailed QC reports, and expert consultation to drive success.
Discover How We Can Help - Request a Consultation
Adeno-Associated Virus (AAV) vectors lead in in vivo gene therapy, providing a safe, effective means to deliver therapeutic genes. Decades of research confirm their non-pathogenicity and long-term gene expression from a single administration. A leading journal's meta-analysis identifies AAV as the most potent gene transfer vehicle, with over 136 ongoing clinical trials validating its efficacy and safety.
AAV Design for Gene Replacement
Mechanism
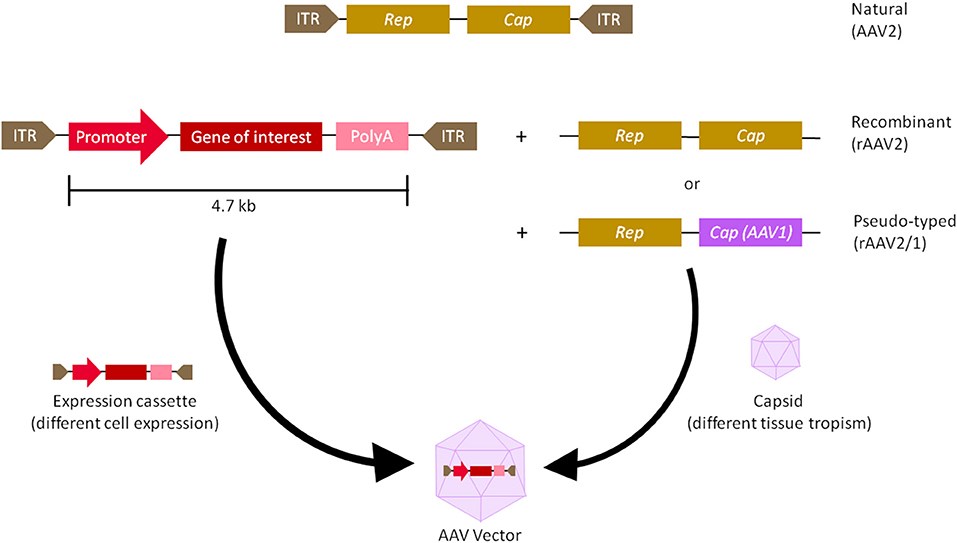 Fig.1 Genomic diagrams of wild and recombinant AAV. The viral genome of recombinant AAV has synthetic expression boxes containing promoters, transgenes of interest, and terminators, such as the polyA sequence, with ITRs on both sides.1
Fig.1 Genomic diagrams of wild and recombinant AAV. The viral genome of recombinant AAV has synthetic expression boxes containing promoters, transgenes of interest, and terminators, such as the polyA sequence, with ITRs on both sides.1
AAVs are small, non-enveloped viruses precision-engineered for gene therapy. Recombinant AAVs (rAAVs) are constructed by replacing viral genes (rep and cap) with a therapeutic expression cassette, which includes a promoter, target gene, and polyA signal. Upon delivery to target cells, rAAVs uncoat, and their single-stranded DNA genomes convert to double-stranded molecules that remain as stable episomes in the nucleus.
Application
The versatility of AAVs allows for a broad range of therapeutic applications. As highlighted in a case study, AAVs are particularly well-suited for gene replacement therapy in monogenic diseases, especially those affecting the central nervous system, such as Spinal Muscular Atrophy and Canavan disease. They are also widely used in ophthalmology, hematology, and muscle disorders. The ability to engineer AAV capsids with specific tropism allows for targeted delivery to tissues like the retina, liver, or brain.
Advantages
AAV vectors offer several key advantages that make them the preferred choice for gene therapy. They are known for their exceptional safety profile and their low immunogenicity compared to other viral vectors. Importantly, AAV vectors generally remain as episomes in the nucleus, minimizing the risk of insertional mutagenesis. Their ability to achieve durable, long-term expression from a single administration provides the potential for a one-time curative treatment for many chronic diseases, which is a major advantage for both patients and the healthcare system.
Workflow
-
Required Starting Materials: To initiate your project, we require a few key pieces of information to ensure the most effective design:
- The gene of interest (GoI) sequence.
- The target cell type or tissue for gene delivery (e.g., hepatocytes, motor neurons, retinal cells).
- Your desired expression profile (e.g., constitutive, tissue-specific, inducible).
- Target Analysis & Strategy Design:Analyze project goals; review GoI, target cell, and disease mechanism to design customized AAV strategy (serotype, promoter selection).
- Transgene Cassette Optimization: Engineer cassette (promoter, GoI, polyA) for robust long-term expression, adhering to ~4.7 kb AAV packaging limit.
- Capsid Engineering and Serotype Selection: Select/modify serotypes for target tropism (e.g., AAV9 for CNS, AAV8 for liver); optimize to reduce immunogenicity.
- Vector Production and Purification: Produce high-titer/purity AAV via advanced purification, removing impurities and empty capsids.
- Quality Control and Validation: Rigorous QC (titer, purity, sterility, in vitro transduction); in vivo validation available upon request.
-
Final Deliverables:
- Report on vector design, production, and QC data.
- Certificate of analysis for key attributes (titer, purity, etc.).
- High-titer AAV vector for research/clinical use.
- Estimated Timeframe: 8–14 weeks, dependent on project complexity (target novelty, capsid modifications, production scale).
What We Can Offer
At Creative Biolabs, we are committed to being your comprehensive partner in gene therapy. Our approach goes beyond standard services to provide tailored solutions and unparalleled expertise for your unique project needs.
Customized, End-to-End AAV Services
One-stop platform spanning strategy design to vector delivery, ensuring smooth execution and uniform quality.
Advanced Capsid Engineering
Engineer novel capsids (not just off-the-shelf serotypes) for optimized tropism and reduced immunogenicity to overcome delivery challenges.
Robust Transgene Cassette Optimization
Meticulous optimization for maximum expression and durability, accelerating therapeutic development.
Scalable and GMP-Ready Production
State-of-the-art facilities enable scale-up from research to clinical-grade, supporting confident IND submission.
Uncompromising Quality Assurance
Adopt QbD framework and rigorous PAT to meet top standards for purity, titer, and efficacy.
Comprehensive Quality Control
Use high-standard tools to quantify/evaluate product aspects (sterility, in vitro transduction efficiency, etc.).
Expert Consultation
Experienced scientists offer ongoing support—from serotype selection to regulatory navigation.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
What are the key factors to consider when choosing an AAV serotype?
Core factors include target tissue tropism, pre-existing neutralizing antibodies in patients, and vector immunogenicity. Our experts help select the optimal serotype for your needs.
How does Creative Biolabs handle the challenge of the host immune response?
We address it via careful serotype selection, pre-existing antibody screening, and advanced capsid engineering to develop less immunogenic vectors for long-term efficacy.
How is the quality and purity of your AAV vectors ensured?
Through multi-stage purification, advanced production techniques, and comprehensive assays (titer, purity, sterility) to meet high standards.
Can you accommodate my specific gene of interest, even if it's a large gene?
Yes—we use strategies like truncated functional genes or dual-vector systems (split/recombined in vivo) to work within AAV's packaging limits.
How do you support projects from preclinical research through to clinical trials?
We provide preclinical vectors, seamless GMP-scale production for trials, and expert support to navigate regulations and accelerate development.
Creative Biolabs is your trusted partner for custom AAV Design for Gene Replacement. Our team of experienced scientists and advanced technological platforms is ready to help you overcome the challenges of gene therapy and bring your therapeutic vision to life.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Au, Hau Kiu Edna, Mark Isalan, and Michal Mielcarek. "Gene therapy advances: a meta-analysis of AAV usage in clinical settings." Frontiers in medicine 8 (2022): 809118. https://doi.org/10.3389/fmed.2021.809118. Distributed under Open Access license CC BY 4.0, without modification.
