AAV Vector Design Service for Gene Addition
Introduction
Our custom AAV Design for Gene Addition service addresses long therapy development cycles, unstable transgene expression, and targeting challenges. Through advanced vector engineering and high-throughput screening, we supply tailored, precision-optimized AAV vectors. Optimized for maximum expression, minimal immunogenicity, and tissue specificity, these vectors support preclinical/clinical studies, backed by comprehensive quality and function analysis.
Discover How We Can Help - Request a Consultation
Gene therapy holds great promise for treating genetic diseases by delivering therapeutic genes to target cells. AAV has become the preferred vector for this purpose, owing to its non-pathogenic nature and capacity to achieve long-term gene expression without integrating into the host genome. Recent literature indicates that AA's effectiveness depends heavily on a customized approach, including vector design and specific serotype selection, with personalized medicine and modular engineering principles being critical to success in the field.
AAV Design for Gene Addition
Mechanism
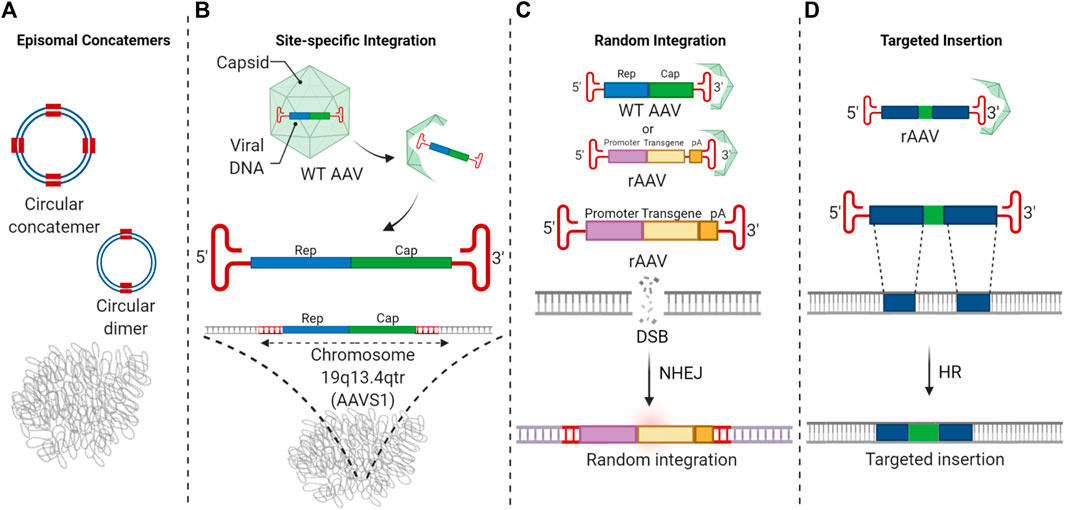 Fig.1 The recombinant AAV vector genome is integrated into the target cell genome, and the target gene is inserted.1
Fig.1 The recombinant AAV vector genome is integrated into the target cell genome, and the target gene is inserted.1
Adeno-associated viruses (AAVs) are small, non-enveloped viruses that are non-pathogenic in humans. Our service uses recombinant AAVs (rAAVs), which have had their viral genes replaced with a therapeutic gene of interest. The rAAV vector is delivered to the target cell, where it uncoats and travels to the nucleus. Here, the single-stranded DNA genome is converted to a double-stranded episome, which then acts as a template for long-term protein expression. This process is highly efficient and safe, as the vector remains outside the host's chromosomal DNA.
Application
AAV design for gene addition has broad applications across a variety of therapeutic areas. It is particularly effective for:
- Rare Genetic Disorders: Correcting a defective gene responsible for diseases like spinal muscular atrophy and hemophilia.
- Neurological Diseases: Delivering therapeutic genes across the blood-brain barrier to treat conditions such as Parkinson's disease and Huntington's disease.
- Ocular Diseases: Restoring vision in inherited retinal dystrophies.
- Cardiovascular and Liver Disorders: Delivering genes capable of restoring proper function, with applications in treating numerous congenital and acquired conditions.
Advantages
The advantages of AAV as a gene delivery vehicle are significant. This vector is renowned for its outstanding safety performance and capacity to transduce both dividing and non-dividing cells, thereby enabling stable, long-term transgene expression. AAVs also exhibit low immunogenicity compared to other viral vectors, minimizing the risk of a strong immune response that could clear the therapeutic vector and its cargo.
Workflow
-
Required Starting Materials: To initiate your project, we require:
- The sequence of your target therapeutic gene.
- Your desired expression profile and target cell or tissue type.
- Any existing data on host immune response or neutralizing antibodies.
- Initial Consultation and Project Scoping: In-depth discussion to clarify therapeutic goals, timeline, and requirements; review starting materials, propose customized AAV design strategy; outcome: defined project plan.
- Vector Design and Optimization: Use proprietary algorithms/databases to design transgene cassettes (promoter, enhancer, polyadenylation signal) and select optimal AAV serotype; outcome: AAV vector blueprint.
- AAV Production and Purification: Produce high-titer AAV in HEK293 cells via bioreactors/triple-plasmid transfection; purify via affinity chromatography/ultracentrifugation; outcome: pure, concentrated AAV.
- Quality Control and Functional Validation: Rigorous QC (titer, capsid purity, transgene integrity) and functional assays (in vitro/in vivo transduction); outcome: fully tested, high-quality product.
- Final Delivery: Deliver purified AAV and supporting documentation; outcome: final product and data for subsequent research.
-
Final Deliverables:
- Purified AAV vector.
- A comprehensive project summary report detailing the design, production, and quality control data.
- A detailed Certificate of Analysis (CoA) confirming vector purity and titer.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 16 weeks, depending on the complexity of the vector design, the scale of production, and the required functional validation assays.
What We Can Offer
At Creative Biolabs, we recognize that every gene therapy project is unique. Our AAV Design for Gene Addition service is not a one-size-fits-all solution; it is a collaborative partnership designed to meet your specific needs. Our comprehensive approach and expertise ensure that you receive a product tailored for success.
Our Advantage
Customized, End-to-End Solutions
One-stop service from concept to vector delivery, covering upstream and downstream process development for seamless, efficient gene therapy research.
Optimized Vector Expression
Specialize in codon optimization for target cell lines; guarantee strain stability in cell banks and large-scale production for consistent performance.
Scalability for All Project Sizes
Adaptable capabilities to meet laboratory-scale or industrial large-scale needs, maintaining consistent quality across volumes.
Uncompromising Quality and Reliability
Implement established quality systems (QbD, PAT), adhere to GMP fermentation standards and strict aseptic verification; use high-standard QC tools for product evaluation.
Comprehensive Documentation and Support
QA-approved strain origin documentation and procedures, ensuring full transparency and regulatory confidence.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How do you ensure the AAV vector will work in my specific cell type?
Our process begins with a detailed analysis of your target cell type. We then use our extensive library of AAV serotypes and a data-driven approach to select the best vector for your application. We can also perform functional validation assays to confirm specificity before final delivery.
What are the key advantages of using your service over producing AAV vectors in-house?
Our service offers significant benefits by providing high-quality, clinical-grade vectors with a faster turnaround time and at a lower cost compared to in-house production. Our expertise in vector optimization and rigorous quality control protocols ensures superior efficacy and consistency.
What precautions should I take when working with the final AAV vector?
Our vectors are produced under strict safety guidelines. We provide a detailed safety data sheet (SDS) and recommendations for handling the vectors. As a general practice, AAVs should be handled with standard biosafety level 1 (BSL-1) precautions in a laboratory setting.
How does your service compare to other gene delivery methods like lentivirus or adenovirus?
While other vectors exist, AAVs offer a superior safety profile, with long-term expression and minimal immunogenicity, making them ideal for many gene therapy applications. Our specialized expertise in AAV engineering allows us to maximize these advantages for your project.
To learn more about our AAV Design for Gene Addition service or to discuss your specific project needs, please do not hesitate to reach out to our team of experts. We are ready to provide the scientific and technical support you need to bring your gene therapy project to life.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Bijlani, Swati, et al. "The role of recombinant AAV in precise genome editing." Frontiers in Genome Editing 3 (2022): 799722. https://doi.org/10.3389/fgeed.2021.799722. Distributed under Open Access license CC BY 4.0, without modification.
