AAV Vector Design Services for Gene Expression
Introduction
Challenges with stable, efficient gene expression can be addressed by our custom AAV Vector Design for Gene Expression service. Leveraging advanced engineering and optimization, we deliver tailored solutions, including end-to-end support for vector design, cloning, and packaging. You'll receive meticulously validated AAV vectors with enhanced tissue tropism, optimized expression, and improved safety, helping overcome off-target effects and poor transduction for reliable research tools.
Discover How We Can Help - Request a Consultation
AAV vectors are a cornerstone of modern gene therapy due to their low immunogenicity and ability to provide stable, long-term gene expression. They are widely used in a variety of research and clinical applications, including the treatment of genetic disorders. Recent literature highlights the importance of vector engineering to improve tissue specificity, overcome packaging limitations, and reduce immune responses, making custom vector design a critical step in any gene therapy project.
AAV Vector Design for Gene Expression
The design of a functional AAV vector is a multi-faceted process that involves engineering several key components. A typical AAV vector contains a transgene cassette flanked by inverted terminal repeats (ITRs). The ITRs are essential for viral packaging and replication. Inside the cassette, a promoter drives the expression of the gene of interest, and a poly(A) signal ensures proper termination of transcription.
Key Components
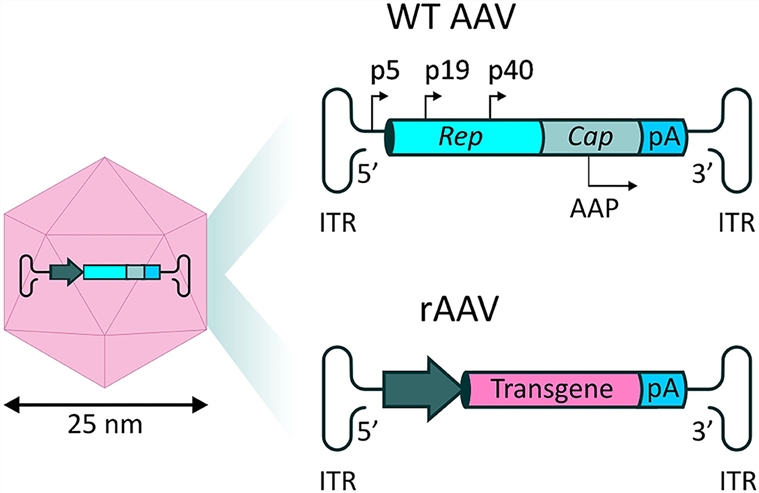 Fig.1 Schematic diagrams of the structures and genomic components of wild and recombinant AAV vectors.1
Fig.1 Schematic diagrams of the structures and genomic components of wild and recombinant AAV vectors.1
- Inverted Terminal Repeats (ITRs)
~145-nucleotide palindromic sequences flanking the vector. As the only virus-derived elements, they mediate genome replication, guide transgene packaging into capsids, and protect DNA for long-term episomal retention in the nucleus.
- Promoter
Regulates transgene expression intensity and specificity. Examples: CMV (broad, high-efficiency for research), MyoD/GFAP (tissue-specific, reducing off-target risks).
- Transgene
Core functional sequence (e.g., micro-dystrophin for DMD, GFP). Restricted by AAV's ~4.7kb packaging capacity—split vectors or truncation are needed for oversized sequences.
- Poly(A) Signal
Downstream sequence (e.g., SV40 polyA) that terminates transcription and adds a polyadenylate tail to mRNA, enhancing stability and nuclear export for efficient translation.
- Enhancer
Synergizes with promoters to amplify transcription (e.g., CMV enhancer for broader expression; muscle-specific enhancers for targeted upregulation).
- Capsid Proteins
VP1/VP2/VP3-composed shell that determines tissue tropism and immunogenicity. Different serotypes have distinct targeting (AAV9: CNS/muscle; AAV8: liver); directed evolution (e.g., AAV2-retro) expands delivery capabilities. Optimization is required to reduce neutralizing antibody responses.
Single Gene Delivery
For most standard applications, a single AAV vector is sufficient to deliver a gene of interest, provided the gene's size is within the 4.7 kb packaging limit. This is the most common and straightforward approach.
Large Gene Delivery
When a gene exceeds the packaging limit, a dual vector system is used. The gene is split into two parts, each packaged into a separate AAV vector. Upon co-transduction, the two fragments recombine to form the full-length, functional gene.
Multiple Gene Delivery
Multiple genes can be delivered using a single AAV vector through the use of a dual-promoter system, where two genes are expressed independently, or with a specific translational element, which allows for the expression of two genes from a single mRNA transcript.
Workflow
- Required Starting Materials: Detailed project description including gene of interest (cDNA sequence), target cell/tissue, and specific promoters/regulatory elements to enable tailored vector design.
- Initial Consultation & Design: Scientific team collaborates to align with project goals, designing a custom strategy including serotype and promoter selection.
- Vector Construction: Gene synthesis and cloning to build a recombinant AAV plasmid with the target gene.
- Virus Packaging & Purification: Vector packaging into AAV particles, followed by high-purity purification of viral stock.
- Titer Determination: Viral particle quantification to ensure high-titer stock for consistent results.
- Quality Control & Validation: Rigorous checks to verify vector integrity and functionality.
- Final Deliverables: Purified high-titer AAV stock, comprehensive report (vector design, QC data), and relevant plasmids.
- Estimated Timeframe: 6-12 weeks, varying by gene complexity and number of serotypes.
What we can offer
Here at Creative Biolabs, we provide a robust and flexible portfolio of AAV products tailored to your research needs, from our off-the-shelf catalog vectors to fully customized solutions for your most complex projects. Our offerings are designed to provide the highest quality and flexibility to accelerate your scientific discoveries.
Customized Vector Design
We optimize the entire vector cassette, including promoter, transgene, and regulatory elements, to meet the specific expression requirements of your target cells and tissues.
Flexible Serotype Selection
Choose from a wide range of AAV serotypes to achieve optimal tissue tropism for your unique in vitro or in vivo applications.
Advanced Gene Handling
Our specialized systems can handle large genes via dual vector systems and multiple genes via a translational element or 2A peptide-based designs, overcoming typical packaging limitations.
High-Titer Production
We guarantee high-titer vector production with concentrations exceeding 1013 GC/mL, ensuring robust and reproducible results in your experiments.
GMP-Compliant Processes
Our production methods adhere to the principles of Good Manufacturing Practice (GMP) to ensure the highest levels of quality, purity, and safety.
Rigorous Quality Control
Every vector batch undergoes comprehensive quality control, including qPCR for titer, silver stain for purity, and sterility testing to ensure superior quality.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How do you choose the right AAV serotype for my project?
We consider your target cell type, tissue, and route of administration. Our team uses a proprietary database of serotype tropisms and can also perform in-house experiments to determine the most effective serotype for your specific application.
Can your AAV vectors be used for both in vitro and in vivo studies?
Yes, our AAV vectors are optimized for high-level expression and efficient transduction in both in vitro cell cultures and in vivo animal models, providing a seamless transition from preclinical research to clinical development.
What measures do you take to ensure the safety of the AAV vectors?
We use a triple-plasmid transfection system to produce our AAV vectors, ensuring they are replication-deficient and non-pathogenic. Our rigorous quality control process includes checks for purity, titer, and the absence of helper viruses.
I have a very large gene. Is it possible to use AAV vectors for my project?
Absolutely. For genes that exceed the AAV packaging capacity, we offer a dual vector system where the large gene is split into two fragments. These fragments are then delivered on separate AAV vectors and recombine within the target cell to form a full-length, functional gene.
Creative Biolabs offers a complete suite of services for AAV vector design and gene expression. From initial consultation to final delivery, we are your partner in success. Our dedication to quality and scientific excellence ensures you receive the best tools for your research.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Marino, Marika, and Matthew G. Holt. "AAV vector-mediated antibody delivery (A-MAD) in the central nervous system." Frontiers in neurology 13 (2022): 870799. https://doi.org/10.3389/fneur.2022.870799. Distributed under Open Access license CC BY 4.0, without modification.
