Chemical Excipients-based Delivery for RNAi
The RNAi mechanism can greatly enrich treatments by providing a platform to combat various diseases that are difficult to cure with conventional therapies. In order to overcome the obstacles in the delivery process and realize the wide potential of RNAi-based therapeutics, various innovative non-viral vectors have been vigorously developed to provide a safer and more effective delivery system.
After more than a decade working on RNAi-based therapies, Creative Biolabs has established a powerful and multi-functional drug development and delivery platform based on proprietary technology and extensive scientific expertise. In addition to the ligand-targeted delivery system, we also provide customized chemical excipients-based delivery systems for RNAi therapy.
Nanoparticles are the most commonly used carriers of RNAi. An ideal nanocarrier can not only addresses the challenges of delivering naked siRNA/miRNA, including its chemical instability, extracellular and intracellular barriers and inherent immune stimulation, but also provide "intelligent" targeted delivery.
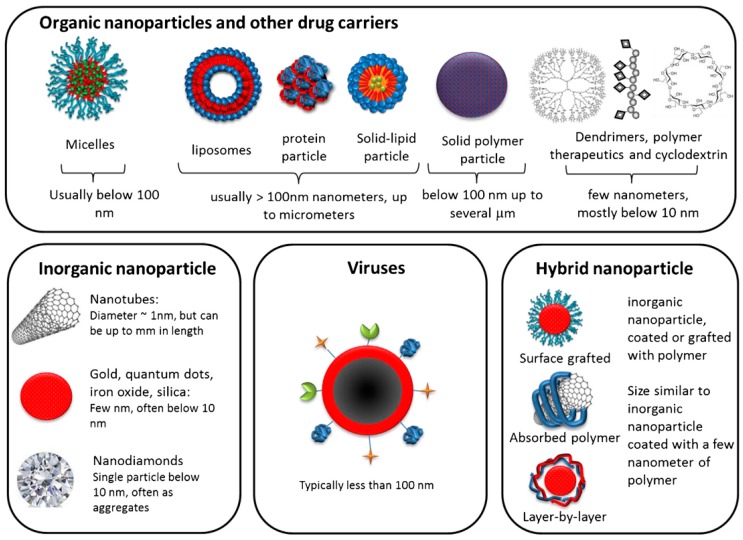 Figure 1. Nanoparticle illustrations that exist in both organic and inorganic. (Tatiparti, 2017)
Figure 1. Nanoparticle illustrations that exist in both organic and inorganic. (Tatiparti, 2017)
Polymer-mediated siRNA delivery has the advantages of facile chemical modification, good biocompatibility, multi-functionality, and integration with inorganic materials, which can address various impediments associated with efficient siRNA delivery. Commonly used polymeric siRNA carriers are polyethyleneimine (PEI), poly(lactic-co-glycolic acid) (PLGA), polypeptide, chitosan, cyclodextrin, dendrimer and polymer-containing different nanoparticles.
Nucleic acid nanotechnology has been developed to be a promising strategy for preparing nano-biomaterials with structural programmability, spatial addressability and excellent biocompatibility. Self-assembled DNA nanostructures can be equipped with functional nucleic acids through DNA hybridization, further serving as a carrier for therapeutic drugs based on RNAi.
Exosomes are a subtype of extracellular vesicles that originate from the endocytic pathway. While being participated in the intercellular transport of macromolecules, exosomes can be used as a system to commute RNA molecules and proteins in the body due to their presence in various body fluids. Therefore, exosomes are considered as promising and effective carriers of small interfering RNA (siRNA), because they exist in the body's endogenous system and have high tolerance.
N-Acetylgalactosamine (GalNAc), an amino sugar derivative of galactose, has also been used to target asialoglycoprotein receptors, specifically for receptor-targeted delivery of siRNA-carrying lipid nanoparticles. Studies have shown that the combination of siRNA with GalNAc-derived asialoglycoprotein receptor ligands can help siRNA target delivery to liver cells in vitro and in vivo. Compared with the maternal, the chemical modification of siRNA has a 5-fold improvement in the efficacy in vivo.
Features of Our Chemical Excipients-based Delivery Structure
- Able to interact with the RNAi molecules, forming protective nanoparticles that can be readily taken up into cells;
- Chemically flexible and amenable to the attachment of tissue-targeted ligands, in vivo stabilizers and other functional sites;
- Can customize a formulation for a specific application and delivery modality.
We believe that these characteristics based on the structure of chemical excipients can provide RNAi with a highly specific delivery system to enhance stability and reduce toxicity in vivo. "Better Targeting, Delivery of RNAi Therapies" is our consistent service tenet. If you are interested in our services, please feel free to contact us.
Reference
- Tatiparti, K.; et al. (2017). siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials. 7(4): 77. Distributed under Open Access license CC BY 4.0, without modification.
