Hypoxia Regulatory Element Targeting Service for AAV Vector
Introduction
For addressing challenges like precise therapeutic gene targeting, drug resistance, and single-dose gene therapy safety/efficacy in cancer treatment, our Hypoxia Regulatory Element Targeting Strategy of AAV Vector delivers payloads exclusively to tumor hypoxic microenvironments via advanced AAV engineering. Creative Biolabs' service offers high specificity for solid tumor-focused projects, using tumor hypoxia to ensure on-site therapeutic gene expression, minimizing off-target effects, maximizing efficacy, and turning hypoxia into a targeting tool for potent, safe treatments with a wider window.
Discover How We Can Help - Request a Consultation
Hypoxia Regulatory Element Targeting Strategy of AAV Vector
Hypoxia-Responsive Elements (HRE)
Hypoxia-Responsive Elements (HREs) are DNA sequences that function as molecular switches. They are activated by the transcription factor HIF-1α, which is overexpressed in hypoxic conditions. When HIF-1α binds to the HRE, it triggers the expression of genes downstream of it. By placing the HRE in front of the therapeutic gene within the AAV vector, we ensure that the gene is expressed only in the low-oxygen environment of the tumor. This mechanism is a key factor in achieving high levels of specificity.
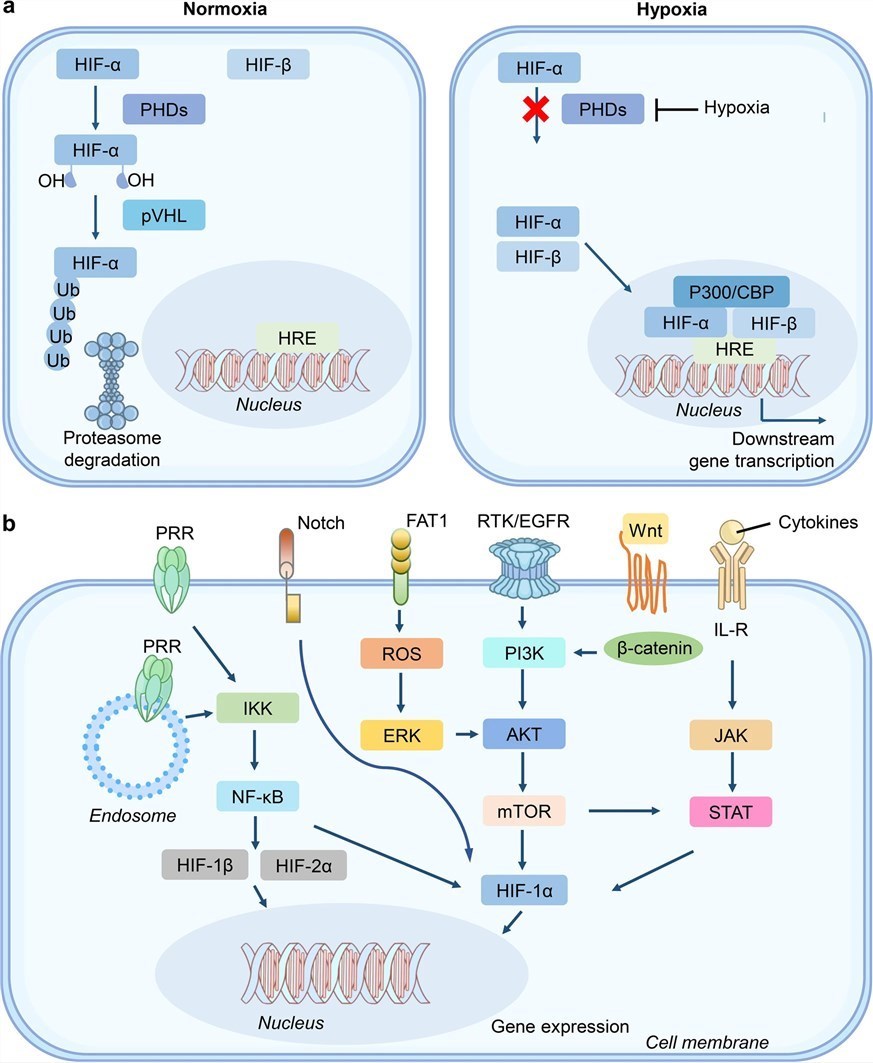 Fig.1 The biological functions involved in hypoxia signaling and its related genes.1
Fig.1 The biological functions involved in hypoxia signaling and its related genes.1
- Classic HRE core sequences
Composed of repeated "RCGTG" motifs (e.g., 5'-TACGTGCT-3'), they directly bind HIF-1α/β heterodimers and are widely used in hypoxia-responsive expression cassettes.
- Derived composite HRE elements
Multiple HRE cores are tandemly linked with a minimal promoter (e.g., CMV) to boost hypoxia-induced specificity and transcriptional activity.
- Tissue-specific HRE combination elements
HRE integrates with tissue-specific sequences (e.g., tumor promoters) for "hypoxia and tissue" dual targeting. Fusion of HRE with hTERT/survivin promoters is used in tumor-targeted therapy.
The Regulation of HREs by the AAV Vector
- HRE Sequence Optimization
Tandem multiple HRE cores (e.g., 3×HRE, 5×HRE) or screen high-affinity variants to strengthen HIF-1α binding, elevating hypoxic transcriptional activation and cutting normoxic leaky expression.
-
Composite Promoter System
Pair HRE with other elements for dual/multiple regulation:- Hypoxia and tissue specificity: Fuse HRE with tumor promoters (e.g., hTERT, CEA) to trigger gene expression (e.g., anticancer genes) solely in hypoxic tumor cells, lessening normal tissue effects;
- Hypoxia and minimal promoter: Link HRE to minimal promoters (e.g., CMV) to avoid excess basal activity, activating only via HIF-1α under hypoxia for greater precision.
- Synergy with Negative Regulatory Elements
Add hypoxia-repressed elements to form a "dual switch" with HRE: ODD represses transcription in normoxia; hypoxia lifts ODD repression and activates HRE, boosting specificity.
- Integration with Inducible Expression Systems
Combine HRE with external systems for "hypoxia plus drug" control: Hypoxia starts TRE basal expression, with drugs adjusting levels for flexible spatiotemporal control of therapeutic genes.
Workflow
- Required Starting Materials: To initiate a project, clients typically provide us with the following:
- Target Gene of Interest: The therapeutic gene(s) to be delivered (e.g., pro-apoptotic genes, immunomodulatory genes, or suicide genes).
- Target Tumor Type: Information about the specific cancer type(s) to be targeted.
- Relevant Biological Data: Any existing data on the expression of the target gene or the hypoxic conditions of the tumor model.
- Project Initiation & Design: We consult to grasp your goals, then design custom AAV vectors—incorporating HRE upstream of your target gene—to enable oxygen-sensitive expression.
- Vector Construction: The expression cassette is cloned into a high-capacity AAV backbone, with full-process QC ensuring construct integrity and sequence accuracy.
- Virus Production & Purification: High-titer, clinical-grade AAV is produced in advanced facilities; chromatography-based purification minimizes empty capsids/impurities and reduces immunogenicity.
- In Vitro Validation: Vectors are tested in cell cultures to confirm robust, specific expression under hypoxia and low basal expression under normoxia.
- In Vivo Efficacy Testing: Optional validation in relevant animal models confirms efficacy and targeting precision, providing critical preclinical data.
-
Final Deliverables: Upon project completion, you will receive a comprehensive package, including:
- High-Titer AAV Vector: The final, purified AAV vector ready for your research or clinical studies.
- Detailed Characterization Report: A full report on vector identity, titer, purity, and functional validation data.
- Recommended Usage Protocol: A guide with best practices for handling, storage, and application of the vector.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 14 weeks, depending on the complexity of the project and the scope of required validation studies.
What we can offer
Custom Vector Design
Our experts work with you to custom-design AAV vectors with optimized Hypoxia Regulatory Elements (HREs) to maximize your therapeutic gene expression specifically within the tumor microenvironment.
High-Quality Production
Leveraging our state-of-the-art facilities, we ensure the production of high-titer, high-purity AAV vectors with minimal empty capsids, which is crucial for reducing immunogenicity and enhancing efficacy.
Advanced In Vitro & In Vivo Validation
We offer robust validation services to confirm your vector's specificity and efficacy. We test gene expression under both hypoxic and normoxic conditions and can also validate the vector in relevant animal models to provide you with the preclinical data you need.
Comprehensive Project Support
We provide end-to-end support, from initial consultation and experimental design to final data delivery and protocol recommendations, ensuring a seamless and productive research experience.
Unrivaled Expertise
Our team of experienced scientists understands the intricacies of AAV vector biology and gene therapy. We are committed to translating cutting-edge science into practical solutions for your project.
Experience the Creative Biolabs Advantage - Get a Quote Today
Case Study
| AAV Vector Structure | Western Blot |
|---|---|
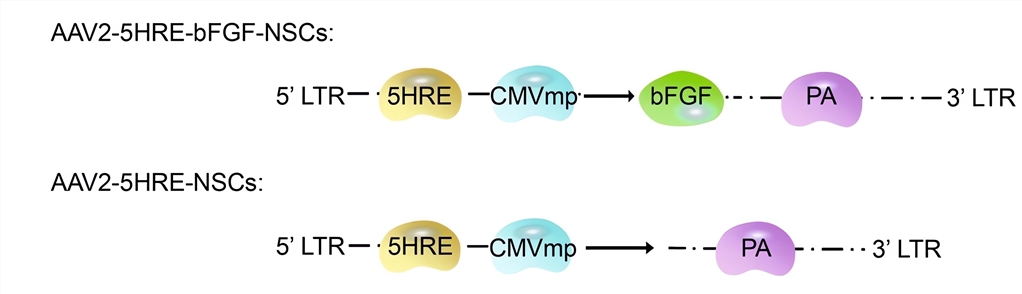
|
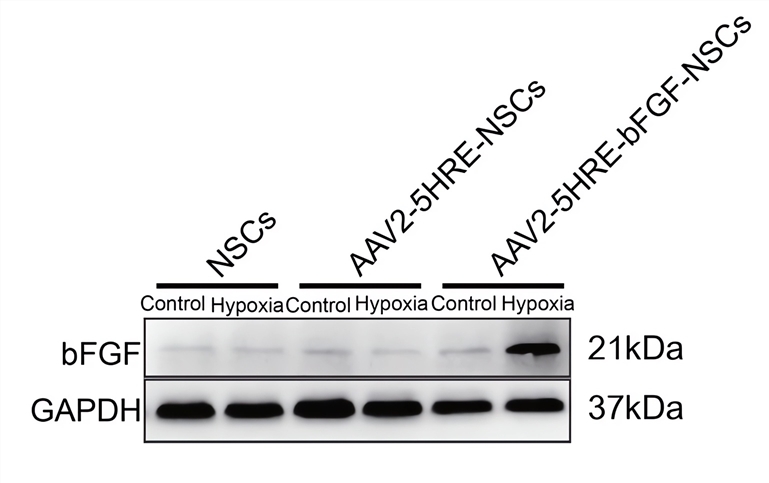
|
| Fig.2 Structural diagrams of AAV2-5HRE and AAV2-5HRE-bFGF.2 | Fig.3 AAV vectors loaded with HREs can promote the expression of target genes in NSCS.2 |
| Inclined Plane Test | BBB Scales |
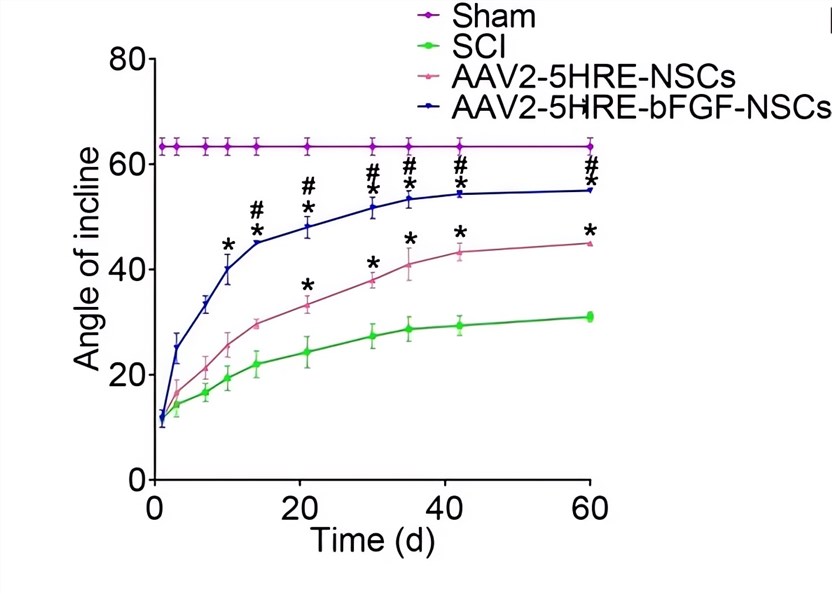
|
|
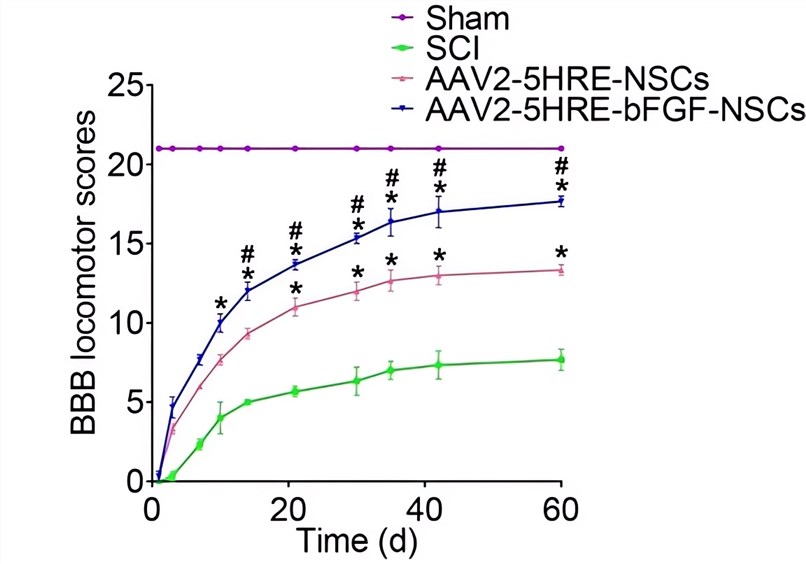
| Fig.4 The inclined plane test results of rats transplanted with AAV-HRE-bFGF-related vectors.2 | Fig.5 The BBB scales of rats transplanted with AAV-HRE-BFGF-related vectors.2 |
| Weight Support | Plantar Step |
|
|
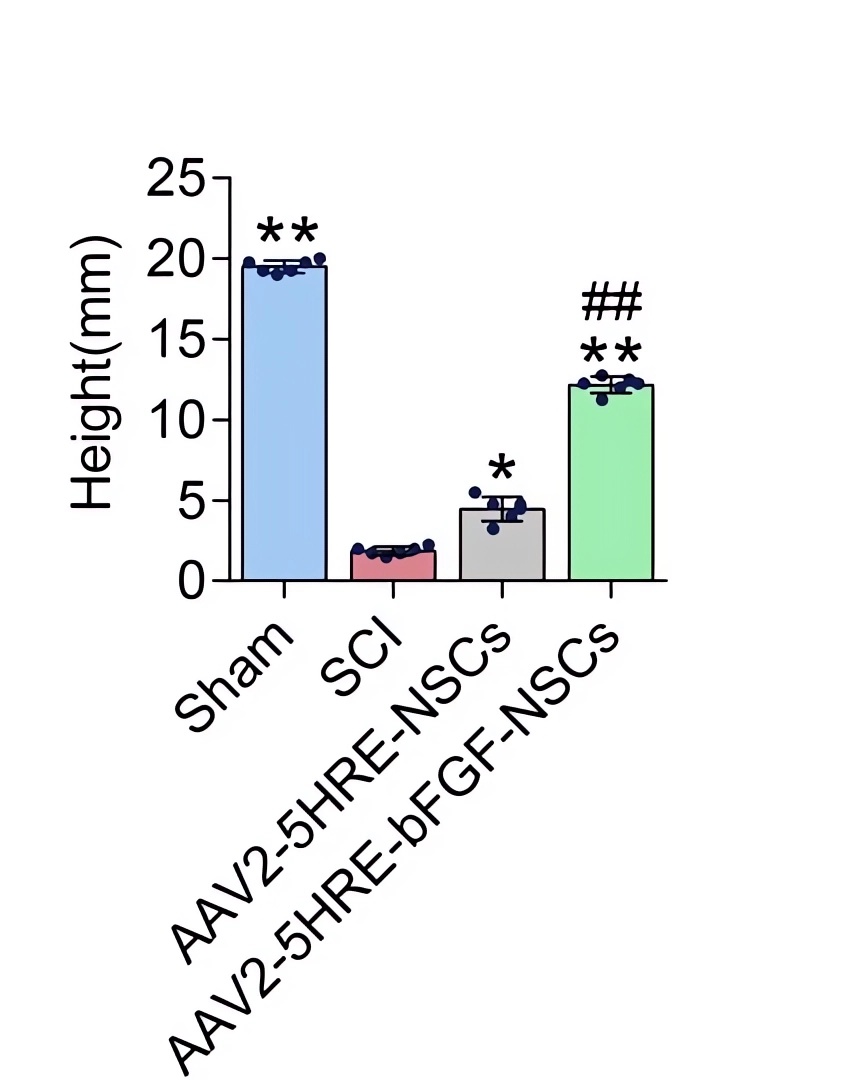
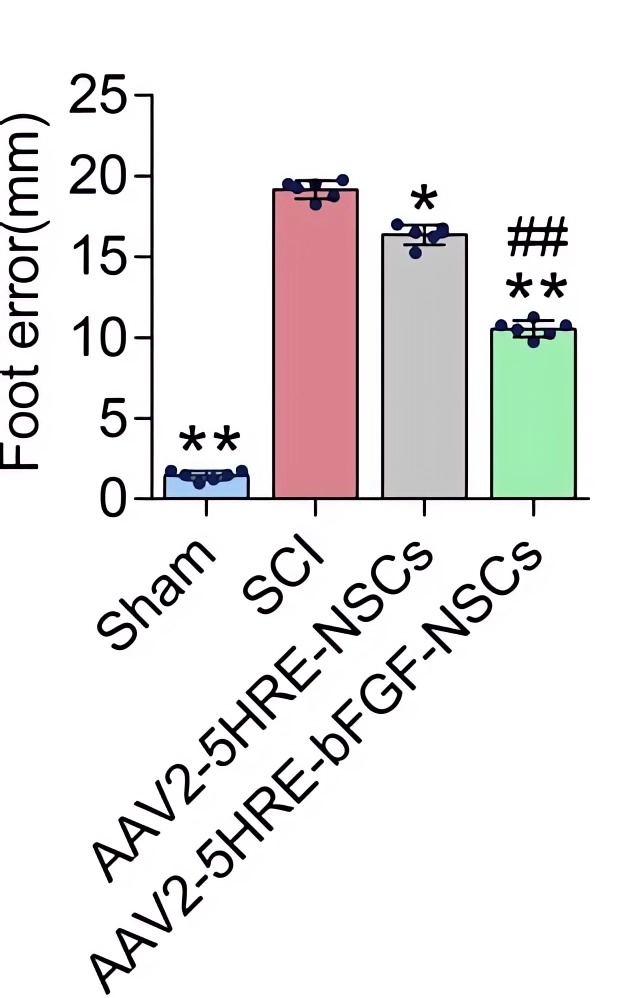
| Fig.6 The hind limb motor ability of rats transplanted with AAV-HRE-bFGF-related vector was significantly enhanced.2 | Fig.7Rats transplanted with AAV-HRE-bFGF-related vectors had less foot error.2 |
| Global Anatomical Observation | |
|
|
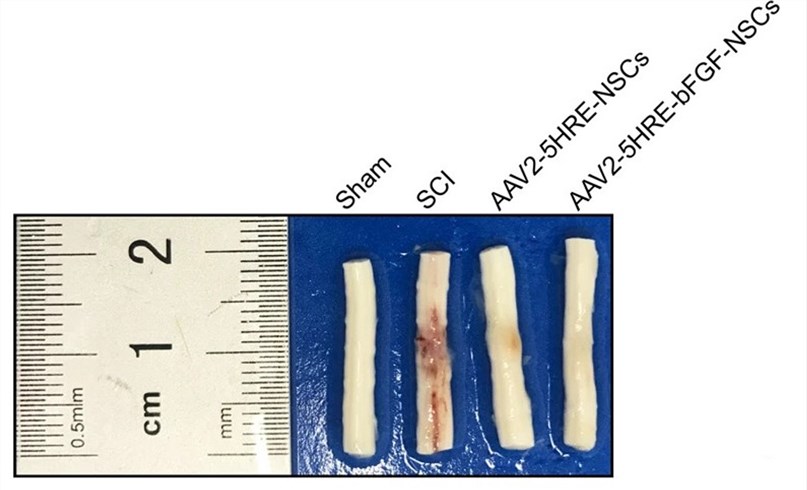
| Fig.8 The rats were dissected, and their spinal cords were observed and compared.2 | |
Customer Reviews

FAQs
How does the Hypoxia-Targeting AAV Vector work in the body?
The AAV vector is designed to circulate until it reaches a target tissue. The therapeutic gene it carries is specifically engineered to be silent until the vector enters a low-oxygen environment, such as a solid tumor. In this environment, a natural cellular switch (HIF-1α binding to the HRE) is flipped, activating the therapeutic gene's expression only in the cancer cells. This process ensures the therapy is localized and effective.
Can this strategy be used for diseases other than cancer?
While the hypoxic strategy is primarily designed for solid tumors, the core technology of targeted gene expression can be adapted for other applications. For instance, it could be relevant for targeting tissues with low oxygen levels due to conditions like ischemia. We encourage you to contact us to discuss your specific project needs and explore potential applications.
Are there any concerns about immunogenicity?
Immunogenicity is a common concern with all AAV vectors due to pre-existing antibodies in the population. While our HRE strategy does not directly mitigate this, our advanced purification methods ensure a product with minimal impurities, which helps reduce the immune response. Furthermore, because our vectors can be effective at lower doses due to their high specificity, the overall immune burden is also reduced.
Creative Biolabs is at the forefront of AAV vector engineering, offering a solution that addresses the critical challenges of specificity and efficacy in gene therapy. Our Hypoxia Regulatory Element Targeting Strategy provides a robust, precise, and safer path for your next-generation cancer therapeutics.
Contact Our Team for More Information and to Discuss Your Project
References
- Luo, Zhen, et al. "Hypoxia signaling in human health and diseases: implications and prospects for therapeutics." Signal transduction and targeted therapy 7.1 (2022): 218. https://doi.org/10.1038/s41392-022-01080-1. Distributed under Open Access license CC BY 4.0, without modification.
- Zhu, Sipin, et al. "AAV2-mediated and hypoxia response element-directed expression of bFGF in neural stem cells showed therapeutic effects on spinal cord injury in rats." Cell death & disease 12.3 (2021): 274. https://doi.org/10.1038/s41419-021-03546-6. Distributed under Open Access license CC BY 4.0, figures were cropped.
