Specific Promoter Driven Targeting Service for AAV Vector
Introduction
For developing therapies for neurological disorders and other hard-to-reach target diseases, Creative Biolabs' Specific Promoter Driven Targeting of AAV Vector Service delivers unparalleled gene delivery precision and efficiency via advanced promoter engineering and viral vector technology. It offers a clear, step-by-step pathway to ensure therapeutic genes are delivered with optimal specificity and efficacy, minimizing off-target effects and maximizing therapeutic potential.
Specific Promoter Driven Targeting of AAV Vector
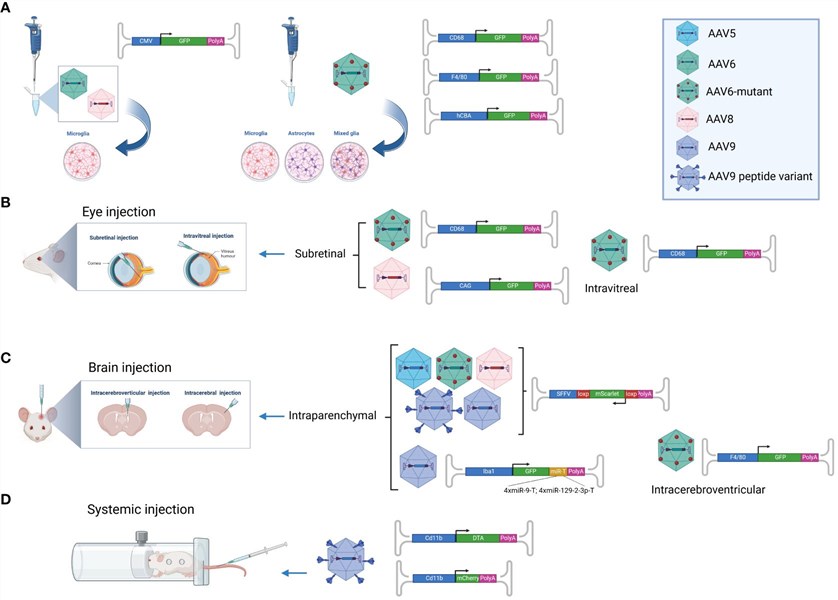 Fig.1 By combining different promoters, the targeting of AAV to microglia is enhanced.1
Fig.1 By combining different promoters, the targeting of AAV to microglia is enhanced.1
Brain resilience relies on neurogenesis, but early-life stress induces chronic neuroinflammation, depleting neural stem cells and impairing brain function. Specific promoter-driven AAV vectors offer a solution: they control gene expression at the cellular level, precisely delivering therapeutic genes to restore neurogenesis, counter inflammation, and aid complex neurological disorders.
AAV Applications Driven by Tissue/Cell-Specific Promoters
- Gene Therapy Field
- Liver Disease Treatment: TBG/hAAT promoter-driven AAV targets hepatocytes, delivering therapeutic genes to express deficient proteins (e.g., coagulation factors) for hereditary liver diseases like hemophilia.
- Cardiac Disease Treatment: cTnT promoter targets cardiomyocytes, delivering genes to repair function (e.g., promoting regeneration, improving contractility) for conditions like myocardial infarction.
- Neurological Disease Treatment: SYN promoter targets neurons (delivering neuroprotective/repair genes for Parkinson's, Alzheimer's); GFAP promoter targets astrocytes for astrocyte-related neurological disorders.
- Diabetes Treatment: Insulin promoter targets pancreatic β-cells, delivering genes to regulate insulin secretion or repair β-cell function.
- Disease Model Construction Field
- Cardiac Disease Models: ANF promoter-driven AAV9-ANF-Cre mediates atrial-specific gene recombination/knockdown, constructing models for studying atrial disease pathogenesis and therapies.
- Neurological Disease Models: Neuron/glia-specific promoters drive target gene overexpression/knockout, building models to study disease onset and progression.
- Basic Research Field
- Cell Function Studies: Specific promoters drive reporter genes to label target cells, aiding observation of cell behavior under physiological/pathological conditions.
- Gene Regulation Mechanism Studies: Genes linked to specific promoters are introduced into target cells to study their expression regulation and impacts on cellular function/phenotype.
Workflow
-
Required Starting Materials: To initiate the service, clients typically provide the following:
- The complete sequence of the therapeutic gene or a clear description of the protein of interest.
- Information on the specific target cell type (e.g., neurons, astrocytes, etc.) and tissue.
- A concise summary of the disease model or therapeutic objective.
- Promoter Engineering and Vector Construction: We engineer custom AAV vectors using our diverse cell-specific promoter library or novel designs, ensuring therapeutic genes are expressed exclusively in target cells to prevent systemic toxicity and ensure efficacy.
- Vector Production and Quality Control: AAV vectors are produced in our advanced facility, with rigorous QC checks (titer, purity, integrity) to meet top standards for research and preclinical use.
- In Vitroand In Vivo Validation (Optional): Optional validation includes dose-response studies and expression analysis in cell cultures or animal models to confirm vector specificity and function.
-
Final Deliverables: Upon project completion, you will receive a comprehensive package.
- The purified, ready-to-use AAV vector.
- Detailed quality control data and a Certificate of Analysis.
- A full report on the vector construction, promoter details, and validation data.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 12 weeks, depending on the complexity of the promoter engineering and the scope of optional validation studies.
Discover How We Can Help - Request a Consultation
What We Can Offer
AAV Vector Design
Our team provides one-stop AAV vector design and construction services, from promoter selection and engineering to final plasmid preparation.
Customization
We specialize in custom AAV vector services tailored to your specific research needs, including novel promoter design for cell-specific targeting.
Experience and Expertise
With over two decades of experience in gene therapy and viral vectors, our experts can guide your project to success with a deep understanding of the intricacies of gene delivery.
Quality Assurance
We adhere to strict quality control standards, including Quality-by-Design (QbD) and rigorous process analytical techniques (PAT), to ensure the highest quality and purity of your final vector.
Scalability
Our production capabilities are scalable, allowing us to support projects from small-scale research to large-scale preclinical studies.
Customer Reviews

Experience the Creative Biolabs Advantage - Get a Quote Today
FAQs
Why is AAV vector targeting so important for CNS applications?
The central nervous system is a highly complex and sensitive environment. Off-target gene expression can lead to unintended side effects and compromise the integrity of your study. Our specific promoter-driven targeting ensures that your therapeutic gene is expressed exclusively in the desired cell population, maximizing efficacy while minimizing risk.
Can you help me select the right promoter for my target cell type?
Absolutely. Our team has extensive expertise in promoter biology and AAV technology. Based on the information you provide about your target cells and therapeutic goals, we will recommend or design the most suitable promoter to ensure optimal results.
How does this service compare to traditional AAV vector production?
While traditional services provide a general-purpose AAV vector, our service is focused on the critical upstream step of promoter engineering. We go beyond simple vector production to provide a complete, validated solution for targeted gene delivery, which saves you time and resources in the long run by preventing off-target effects.
Is there any risk of the promoter losing its specificity over time?
We employ well-characterized and highly stable promoters with a long history of use in gene therapy research. Our rigorous quality control and validation processes ensure that the integrity and functionality of the promoter are maintained throughout the vector's production and storage.
Contact our team for more information and to discuss your project
Reference
- Stamataki, Maria, et al. "Microglia targeting by adeno-associated viral vectors." Frontiers in Immunology 15 (2024): 1425892. https://doi.org/10.3389/fimmu.2024.1425892. Distributed under Open Access license CC BY 4.0, without modification.
