Self-Deleting Lentiviral Vector Service
The field of advanced therapies is rapidly evolving, with lentiviral vectors (LVs) standing as a cornerstone technology for stable gene delivery in both dividing and non-dividing cells. Their application in CAR-T therapies, hematopoietic stem cell engineering, and in vivo gene transfer has been transformative. However, the permanence of conventional lentiviral integration poses potential long-term risks, including insertional mutagenesis, sustained oncogene expression, or undesired immune responses. Self-deleting lentiviral vectors represent the next evolutionary step, offering the high transduction efficiency of LVs with an added layer of safety through programmable vector excision. This innovative technology enables transient, high-level gene expression followed by the removal of the viral backbone, mitigating risks and opening new avenues for therapeutic applications where temporary genetic modification is desired or essential.
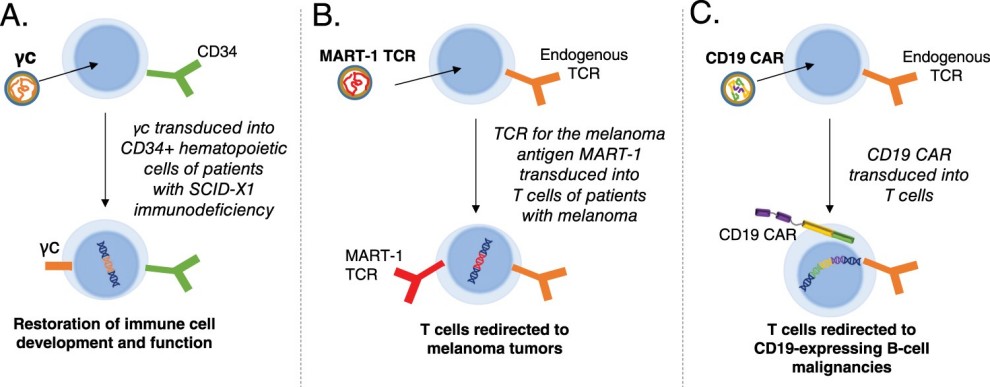 Fig.1 Key clinical applications of lentiviral vectors vector 1,2
Fig.1 Key clinical applications of lentiviral vectors vector 1,2
At Creative Biolabs, we are at the forefront of this innovation. Our comprehensive Self-Deleting Lentiviral Vector Service is designed to be your strategic partner, providing the expertise and robust infrastructure needed to translate your pioneering research into safe, effective, and clinically viable therapies. We empower you to navigate the complexities of vector design and production with confidence, accelerating your path from discovery to pre-clinical development.
Our Integrated Service Workflow: A Partnership for Success
We believe that transparency and rigorous quality control are paramount. Our phase-gated, collaborative process ensures your project remains on track and meets the highest standards at every milestone.
- Strategic Consultation & Project Design: Your journey begins with a deep-dive consultation with our scientific team. We work to understand your therapeutic goals, target cells, and desired expression kinetics. Based on this, we recommend the optimal self-deletion mechanism (e.g., Cre-LoxP, piggyBac transposase, or CRISPR/Cas-based systems), promoter system (ubiquitous, tissue-specific, or inducible), and pseudotyping strategy to achieve precise tropism.
- Vector Design & Bioinformatics: Our bioinformaticians take the lead in computationally optimizing your gene of interest (GOI) for enhanced expression. We strategically design the vector architecture, incorporating all necessary regulatory elements and the chosen self-excision technology into a optimized lentiviral backbone.
- Plasmid Construction & Sequence Validation: Our molecular biology team executes the precise construction of your transfer and packaging plasmids. Every single construct undergoes 100% full-length Sanger sequencing to guarantee perfect sequence fidelity and integrity before we proceed to production.
- Virus Production & Purification: Utilizing our scalable platform based on transient transfection of HEK293T cells, we produce high-titer lentiviral particles. We then employ advanced purification methods, such as ultracentrifugation or chromatographic techniques, to isolate vectors of exceptional purity, effectively removing cellular debris and empty capsids.
- Comprehensive Analytics & Quality Control: This critical phase involves a multi-parameter analysis of your final product. We determine genomic titer (via qPCR/ddPCR), functional titer (using flow cytometry or TCID50 assays), and confirm deletion efficiency in vitro. Rigorous safety testing, including sterility, mycoplasma, and endotoxin assays, ensures the vector is safe for in vivo applications.
- Final Delivery & Documentation: You receive your ready-to-use, high-quality self-deleting lentiviral vector, aliquoted and shipped under optimal conditions. Accompanying the product is a detailed Certificate of Analysis (CoA) and a comprehensive project report, providing full traceability and data for your regulatory files.
What We Do: Core Service Capabilities
Advanced Vector Engineering
Our expertise lies in tailoring the vector to your specific needs. We offer a suite of design options:
- Selection of Deletion Mechanisms: We have extensive experience with various systems. The Cre-LoxP system is ideal for controlled excision upon Cre recombinase expression. The piggyBac system offers precise cut-and-paste transposition, leaving no footprint. For the most advanced applications, we can engineer CRISPR-guided self-deletion systems.
- Promoter and Enhancer Selection: From strong ubiquitous promoters (EF1α, PGK) to tissue-specific (CD4, SYN1) or drug-inducible promoters, we guide you to the choice that maximizes your therapeutic effect while minimizing off-target expression.
- Serotype Pseudotyping: Beyond the standard VSV-G envelope, we can pseudotype with envelopes from Rabies, LCMV, or Ross River Virus to alter cellular tropism, enhance transduction efficiency for specific cell types (e.g., T cells, HSCs, neurons), and evade pre-existing immune responses.
Scalable Vector Production
We support every stage of your development pipeline:
| Grade | Primary Applications | Quality Control & Documentation | Regulatory Support |
|---|---|---|---|
| Research Grade | Initial proof-of-concept studies, target validation, early-stage in vitro/in vivo screening | Standard QC procedures | Not intended for submissions |
| Pre-Clinical Grade | Pivotal toxicology, biodistribution, and efficacy studies | Enhanced QC and rigorous documentation | Supports IND/CTA submissions |
Rigorous Analytical Development
Characterization is key to reproducibility. Our analytics suite includes:
- Titering: Genomic titer (vg/mL) by ddPCR for absolute quantification; functional titer (IU/mL) to measure infectious units.
- Purity and Safety: SDS-PAGE analysis, ELISA for p24 antigen, endotoxin testing (LAL assay), and sterility testing.
- Functional Validation: In vitro assays to quantitatively demonstrate the efficiency and kinetics of the self-deletion event post-transduction.
Key Advantages of Partnering With Us
- Unmatched Expertise: Our team comprises Ph.D.-level scientists with deep domain knowledge in virology, vectorology, and cell therapy, ensuring your project is grounded in sound science.
- End-to-End Integration: We offer a truly seamless service, managing the entire process from sequence to vial under one roof, reducing complexity and streamlining your workflow.
- Commitment to Quality: Our uncompromising adherence to strict Quality Control protocols guarantees that you receive a vector that is not only highly efficacious but also safe and consistent between batches.
- Customization at Every Step: We reject a one-size-fits-all approach. Your project is unique, and our solutions are tailored to your gene of interest, target indication, and clinical objectives.
- Collaborative Partnership: We view ourselves as an extension of your R&D team. Our scientists are available for ongoing consultation, providing insights and troubleshooting support to ensure your success.
Hear From Our Clients
"We needed a sophisticated self-deleting system for our next-generation CAR-T program. The team of Creative Biolabs was phenomenal—their guidance on vector design was invaluable, and the final product exceeded our expectations in both titer and deletion efficiency. Their rigorous QC gave us the confidence to move directly into our pre-clinical models."
— Dr. Sophia Green, Head of Research, Biotech Innovations Inc.
"As an academic lab, navigating the complexities of lentiviral vector engineering can be daunting. Creative Biolabs provided an exceptional end-to-end service. The communication was clear and consistent, and the comprehensive report and CoA made it incredibly easy to include the data in our grant applications. A truly collaborative and expert partner."
— Prof. Ben Carter, Principal Investigator, Institute of Molecular Medicine
"Advancing our regenerative medicine program required a vector that could provide robust but transient expression. We partnered with Creative Biolabs to develop a custom self-deleting lentiviral construct for in vivo delivery. Their platform delivered exceptionally high titers, and the precise deletion kinetics were crucial for achieving therapeutic efficacy without long-term genomic alteration. The entire process, from design to delivery, was seamless and scientifically rigorous."
— Dr. Alex Thompson, Director of Regenerative Medicine
Frequently Asked Questions
Q: What are the primary applications for self-deleting lentiviral vectors?
A: Their key application is in any field where temporary genetic modification is advantageous. This includes:
- Cell Immunotherapy: Engineering CAR-T or TCR-T cells where removing the viral vector after successful tumor killing can improve long-term safety.
- In Vivo Gene Therapy: Delivering therapeutic genes (e.g., for regenerative medicine) where transient expression is sufficient and safer.
- Induced Pluripotent Stem Cell (iPSC) Generation: Reprogramming somatic cells where the permanent presence of reprogramming factors is unnecessary and potentially oncogenic.
- Functional Genomics: Performing transient genetic screens or differentiation protocols.
Q: How do I choose between Cre-Lox, piggyBac, or a CRISPR-based system?
A: The choice depends on your application. Cre-LoxP offers tight temporal control but requires subsequent delivery of Cre recombinase. PiggyBac provides seamless excision without a footprint and is highly efficient. CRISPR-based systems are highly flexible and can be designed to be inducible. Our team will consult with you to determine the most effective and simplest system for your specific goals.
Q: What starting materials do I need to provide?
A: We offer maximum flexibility. You can provide us with the sequence of your gene of interest, and we will handle the rest: codon optimization, synthesis, and cloning. Alternatively, if you have a validated plasmid, we can use that as a starting material upon confirming its sequence.
Q: Can you produce clinical-grade (GMP) self-deleting lentiviral vectors?
A: While our current pre-clinical grade is produced with IND-enabling documentation and quality, we have a clear pathway to GMP manufacturing. We can discuss your clinical production needs and develop a tech transfer plan to our GMP facilities when you are ready to advance.
Empower Your Next Breakthrough
Self-deleting lentiviral technology is redefining the safety and applicability of advanced therapies. Partnering with a team that possesses the scientific depth, technological capability, and commitment to quality is critical to harnessing its full potential.
Let us help you de-risk your development pathway and accelerate your program with a vector platform designed for both efficacy and safety.
Contact us from now to schedule a detailed technical consultation with our experts and receive a personalized project plan.
References
- Milone, M.C., O'Doherty, U. Clinical use of lentiviral vectors. Leukemia 32, 1529–1541 (2018). https://doi.org/10.1038/s41375-018-0106-0
- Distributed under Open Access license CC BY 4.0, without modification.
