Electrospun Polymeric Nanofibers-Based Delivery Strategy: Mechanism, Benefits, and Biomedical Advances
Transdermal and localized drug delivery has long struggled with skin barrier limits and uneven release. Compared with traditional transdermal drug delivery, electrospun polymeric nanofibers (EPNFs) emerge as a game-changer, as their tailored structure, high permeability, and biocompatibility redefine effective biomedical delivery. In this review, Creative Labs will take you through the mechanisms, benefits and biomedical applications of EPNF-based delivery systems.
Introduction to Electrospun Polymeric Nanofibers
Why Transdermal Drug Delivery Needs a New Strategy?
Transdermal drug delivery (TDD), as a non-invasive pharmacotherapy method, has been widely valued for its sustained drug efficacy and minimized systemic toxicity. However, in traditional transdermal drug delivery strategies, such as ointments, traditional patches, and gels, several unavoidable barriers exist to undermine the drug efficacy. On the one hand, a dense barrier formed by the stratum corneum of the skin could block the passage of most drug molecules. On the other hand, an unsteady drug release (either a burst release in initial use or an insufficient long-term release) would also cause therapeutic inaccuracy. Moreover, some carriers have poor biocompatibility, which increases the risk of triggering skin irritation. These drawbacks create an urgent need for a new TDD strategy that can overcome skin barriers, enable precise and controlled release, and enhance biosafety.
What Are Electrospun Polymeric Nanofibers?
Electrospun polymeric nanofibers (EPNFs) stand out as a transformative solution to overcome the limitations of traditional TDD. It is produced via electrospinning—a technique that utilizes high-voltage electric fields to stretch polymer solutions into ultrafine fibres (50 nm–5 μm in diameter) (Figure 1). Upon collection, the fibres form a nonwoven mat that acts as a flexible and breathable vehicle for drugs. Commonly used polymers include polyvinyl alcohol (PVA), polycaprolactone (PCL), PLGA, and zein (a natural corn protein). These polymers confer composed EPNFs with customizable degradation rates, mechanical strength, and biocompatibility for both hydrophilic and hydrophobic drugs. In addition, the porous structures with a high surface area-to-volume ratio in EPNFs enable efficient drug loading and facilitate skin respiration and drug diffusion. Moreover, the morphology (e.g., core-shell, aligned, porous) of EPNFs is customizable (e.g., core-shell, aligned, porous). Compared to hydrogels or microneedle systems, electrospun mats enable rapid drug loading, precise control over release kinetics, and non-invasive administration, eliminating the pain and residue associated with traditional patches. Furthermore, many recent relevant studies suggest that EPNFs not only resolve TDD's key challenges but also drive biomedical advances, making them a core focus in advanced drug delivery studies.
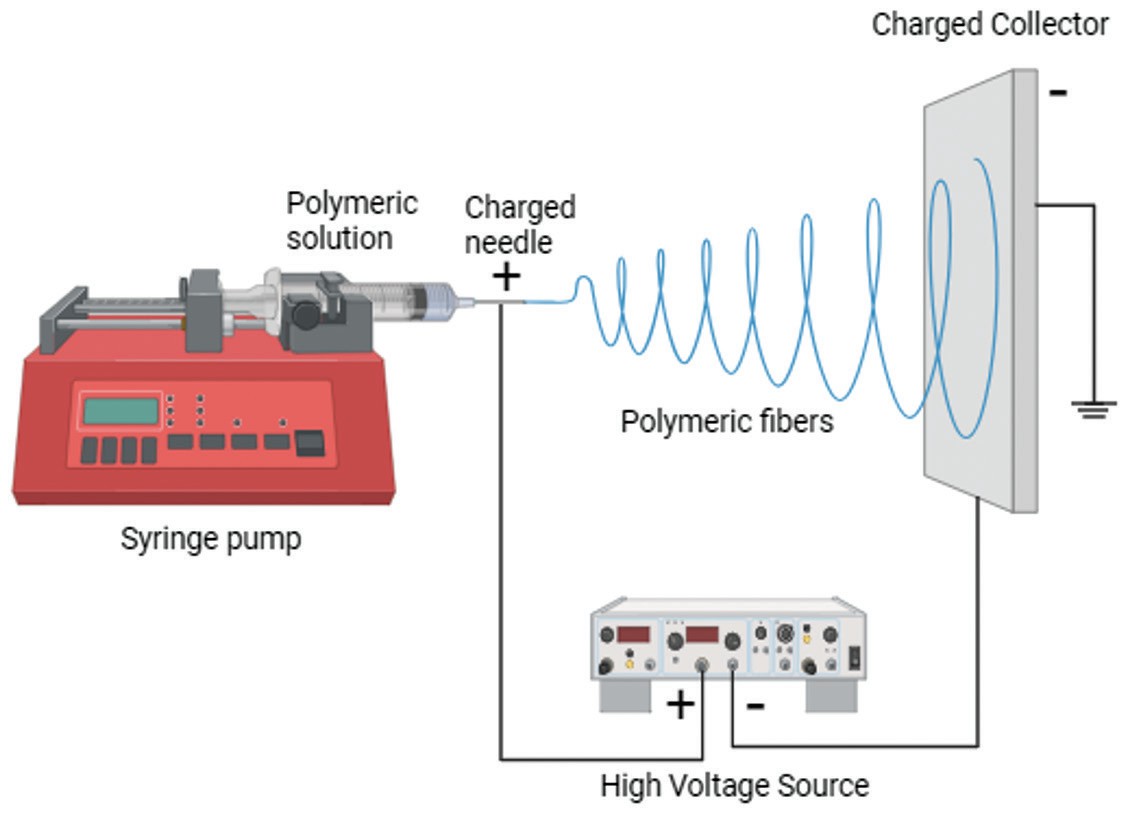 Fig
1 Production of electrospun polymeric nanofibers2.
Fig
1 Production of electrospun polymeric nanofibers2.
Mechanisms of Drug Release and Skin Penetration of EPNFs
Electrospun polymeric nanofibers (EPNFs) achieve effective drug release and skin penetration through three core mechanisms, which are tied to their structural and material traits. This trio of mechanisms ensures EPNFs balance rapid onset and sustained efficacy for transdermal delivery.
Rapid dissolution
Rapid dissolution is enabled by the ultra-high surface area-to-volume ratio and porous nonwoven mat structure of EPNFs. These structural features enable increased exposure of drug-loaded surfaces to skin moisture, leading to the dissolution of encapsulated drugs, especially when paired with hydrophilic polymers (e.g., PVA).
Diffusion
Diffusion drives both drug release from fibres and skin penetration. The porous structure of EPNFs allows therapeutic molecules to diffuse through the fibres. The alignment of the nanofibers with skin microstructures enables encapsulated drugs to permeate the stratum corneum, thereby bypassing the dense skin barrier.
Controlled release
Controlled release relies on polymer selection and fibre design. For example, as synthetic polymers, such as PCL, degrade slowly, they can be used for long-term drug release. Additionally, core-shell fibres can utilize the shell to delay core drug diffusion and regulate the drug release rates.
Properties: Safety, Stability, and Skin Compatibility of EPNFs
Safety remains central to clinical translation. Numerous studies have confirmed that EPNFs are non-irritating, biocompatible, and mechanically stable for prolonged skin contact.
Safety
In terms of safety, EPNFs utilize biocompatible polymers (e.g., FDA-approved PCL and PLA), whose degradation products (lactic acid and caproic acid) can be metabolized by the body without toxic accumulation, and natural polymers (e.g., gelatin or collagen) to minimize skin irritation.
Stability
Regarding stability, EPNFs are able to maintain structural and drug-loading integrity under physiological conditions. For example, PCL-based EPNFs can retain fibre morphology for weeks under physiological conditions, preventing premature drug leakage. In the administration of doxorubicin (a type of sensitive drug), the core-shell configurations of EPNFs are proven to be able to shield sensitive pharmaceuticals (e.g., doxorubicin) from degradation, thereby ensuring that over 80% of the drug remains stable for 30 days.
Compatibility
Regarding skin compatibility, the flexibility and porosity of EPNFs help mitigate adverse skin interactions. EPNF mats can conform to skin contours without causing friction-induced redness; meanwhile, their porous structure enables skin respiration, preventing moisture buildup that can cause discomfort. Additionally, EPNFs coated with collagen can enhance keratinocyte adhesion, thereby supporting skin integration rather than triggering rejection responses.
Design Parameters That Drive Drug Permeation
The performance of electrospun nanofibers depends on several controllable physical parameters, including fiber diameter, polymer hydrophilicity, fiber morphology and nonwoven mat porosity (Table 1). By adjusting these physical parameters, researchers can optimize the drug permeability.
Table 1 The physical parameters of drug permeability.
| Parameter | Key Role in Drug Permeation | Specific Examples |
|---|---|---|
| Fiber Diameter | Aligns with skin microstructures, minimizes gaps between fibres and stratum corneum, enhances drug-skin contact. |
1. 244 nm-diameter PLLA nanofibers (boosted permeation vs. thicker microfibers); 2. 50nm–5μm diameter range (matches skin microstructures) |
| Polymer Hydrophilicity | Tailors drug solubility in skin moisture and balances dissolution rate and structural stability for sustained permeation. |
1. Hydrophilic PVA nanofibers (loaded with gentamicin, quick dissolution to enhance antibiotic
permeation); 2. Gelatin/PCL blends (hydrophilic gelatin+hydrophobic PCL, enabled sustained permeation of curcumin) |
| Fiber Morphology | Creates permeation channels (porous structure) to accelerate drug diffusion, or regulates permeation rate (core-shell structure) to avoid burst release. |
1. Porous silica nanofibers (800 nm diameter-internal channels accelerated drug diffusion); 2. Core-shell PCL/gelatin fibres (gelatin shell controlled permeation rate of core-loaded doxorubicin). |
| Nonwoven Mat Porosity | Enables skin respiration and unobstructed drug diffusion, preventing moisture buildup that blocks permeation. | PAN nanofiber mats enable long-term transdermal applications. |
Manufacturing Scale-Up and Regulatory Pathways of EPNFs
Electrospun polymeric nanofibers (EPNFs) face two key hurdles for EPNF commercialization: manufacturing scale-up and regulatory compliance. Corresponding requirements for these two aspects should be met (Table 2).
Table 2 Core requirements for commercialization of EPNFs.
| Category | Core Requirements | Solutions |
|---|---|---|
| Manufacturing Scale-Up | Traditional single-nozzle electrospinning has low yield and batch inconsistency. | Adopt multi-jet electrospinning, portable ES devices, and combine with 3D printing. |
| Equipment drive shaft clogging during the mechanical cutting of nonwoven mats. | ||
| Regulatory Pathways | Material safety | Use FDA-approved polymers (e.g., PCL, PLA) with metabolizable degradation products (lactic acid, caproic acid); |
| Process standardization | Standardize parameters like homogenization rpm and voltage to ensure batch consistency; | |
| Clinical data support | Trials of EPNF mats for diabetic foot ulcers (proved wound closure efficacy) |
Emerging Biomedical Applications of EPNFs
Electrospun polymeric nanofibers are not only applied to transdermal biologics, but they are also expanding into diverse emerging biomedical applications, including cancer therapy, wound healing, regenerative medicine, and tissue engineering, by leveraging their tunable, drug-loading, and biocompatible capabilities.
Cancer therapy
In cancer therapy, EPNFs can reduce systemic toxicity by enabling localized and controlled drug delivery. For example, cisplatin-loaded PEO/PLA EPNFs can be applied as vaginal implants to continuously release targeted drugs in cervical cancer tissues (U14 cells). In addition, core-shell EPNFs (e.g., PCL/gelatin) can deliver dual drugs (doxorubicin+photothermal agents) for synergistic chemo-photothermal therapy, inhibiting tumour recurrence post-surgery.
Wound healing and regenerative medicine
EPNFs can accelerate repair by mimicking the extracellular matrix (ECM). For instance, collagen-coated EPNFs can promote full-thickness skin wound closure in diabetic rats by enhancing keratinocyte adhesion and angiogenesis.
Tissue engineering
EPNFs can also be applied for structured tissue regeneration. A typical example is 3D-printed EPNF scaffolds (e.g., PCL/HA), which can restore segmental bone defects by balancing mechanical strength and cell infiltration.
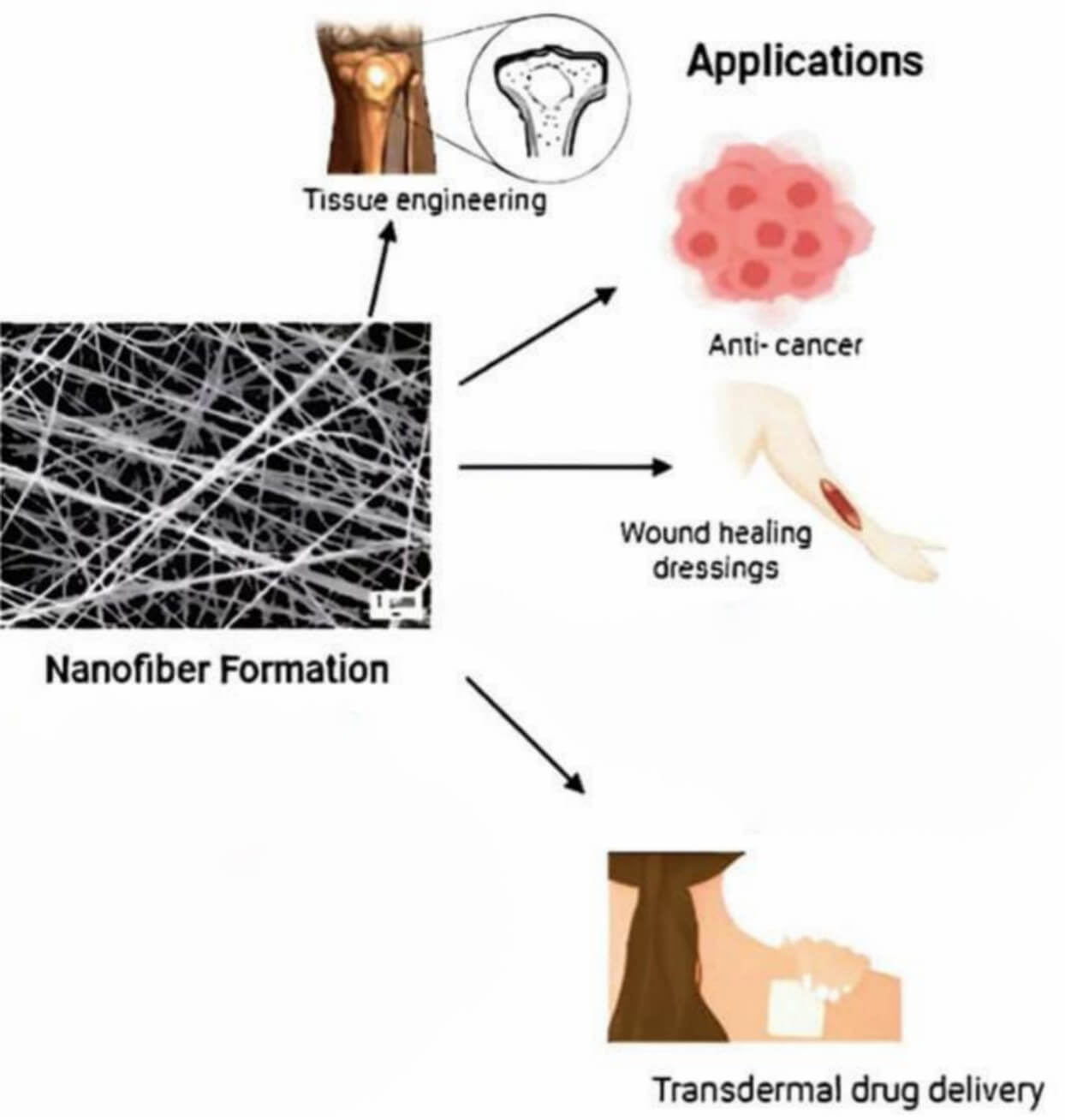 Fig
2 Biomedical applications of EPNFs.2
Fig
2 Biomedical applications of EPNFs.2
Limitations and Practical Troubleshooting of EPNFs
Although electrospun polymeric nanofibers (EPNFs) are beneficial, there are inherent limitations and practical challenges that hinder their application in transdermal delivery (Table 3).
Table 3 Limitations and Troubleshooting of EPNF application in transdermal delivery.
| Key limitation | Description | Troubleshooting |
|---|---|---|
| Fiber agglomeration | Hydrophobic polymers (e.g., PAN, PLLA) tend to clump during fabrication or storage, reducing drug-loading efficiency and skin contact. |
1. Surface modification: oxygen-plasma treatment to enhance the hydrophilicity of PAN/PI
nanofibers; 2. Blending hydrophobic polymers with hydrophilic ones (e.g., PCL/gelatin) to improve dispersion. |
| Inconsistent drug release | Mechanical cutting or sonication of nonwoven mats often results in a wide length distribution of short EPNFs, leading to uneven drug release. | Optimizing processing parameters: controlling homogenization RPM (e.g., 13,000–16,000 rpm for PLA/PCL blends) and sonication pulse mode (2s/2s) to minimize length variation. |
| Poor mechanical stability | The poor mechanical stability of natural polymer-based EPNFs (e.g., gelatin) limits their long-term use. | Core-shell structuring: using PCL as a rigid shell to reinforce gelatin cores, which maintains flexibility while enhancing structural durability. |
Creative Biolabs' Commitment
Creative Biolabs is dedicated to providing advanced electrospun polymeric nanofibers (EPNFs) services related to biomedical delivery for our customers. The related service is listed below:
High-Throughput Electrospinning Screening Platform
Creative Biolabs integrates modular electrospinning systems supporting single- and coaxial fiber production, enabling multi-drug formulations and rapid parameter optimization.
In-House IVPT & Dermatokinetic Modelling
Our dedicated analytical suite quantifies drug flux, lag time, and skin retention within 24 hours—empowering clients to make data-driven decisions faster.
For more related information, please visit our service page and make an appointment with our experts to design the EPNFs for your projects.
Related Services You May Be Interested in
FAQs
Can nanofibers deliver vaccines transdermally?
Yes. Zein nanofibers loaded with H1N1 hemagglutinin can achieve IgG titres which are comparable to intramuscular injection.
What makes electrospun nanofibers a next-generation delivery platform?
They offer ultra-fast dissolution, tunable porosity, and high drug-loading flexibility, surpassing the capabilities of traditional films or patches.
Conclusion
The electrospun polymeric nanofibers-based delivery strategy represents a paradigm shift in transdermal and localized drug administration. With tunable structure, superior skin permeability, and excellent biocompatibility, it offers a viable route for large-molecule and biologic delivery—bridging the gap between convenience and efficacy.
As global interest in EPNF-based delivery systems grows, Creative Biolabs continues to lead innovation with scalable, regulatory-ready solutions that empower our clients to move from concept to experiment through comprehensive formulation, analytical, and regulatory support.
References
- Zelkó, R., Lamprou, D. A. & Sebe, I. "Recent Development of Electrospinning for Drug Delivery." Pharmaceutics 12, 5 (2019). https://www.mdpi.com/1999-4923/12/1/5.
- Kalayil, N., Budar, A. A. & Dave, R. K. "Nanofibers for Drug Delivery: Design and Fabrication Strategies." BIOI 5, (2024). https://scienceopen.com/hosted-document?doi=10.15212/bioi-2024-0023. Distributed under Open Access license CC BY 4.0, without modification
- Elsadek, N. E. et al. Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine. Materials 15, 1934 (2022). https://www.mdpi.com/1996-1944/15/5/1934.
- Shakil, U. A., Abu Hassan, S. B., Yahya, M. Y. & Rejab, M. R. M. "A focused review of short electrospun nanofiber preparation techniques for composite reinforcement." Nanotechnology Reviews 11, 1991–2014 (2022). https://www.degruyter.com/document/doi/10.1515/ntrev-2022-0116/html.
- Dai, X. et al. "Electrospinning based biomaterials for biomimetic fabrication, bioactive protein delivery and wound regenerative repair." Regenerative Biomaterials 12, rbae139 (2025). https://academic.oup.com/rb/article/doi/10.1093/rb/rbae139/7915488.



