Graphene Oxide-based Delivery Strategies: Mechanisms and Applications
In this article, Creative Biolabs provides an overview of the various graphene oxide (GO)-based delivery strategies employed in today's biomedical field.
Introduction to Graphene Oxides (GOs)2–5
Definition of Graphene Oxides
Graphene is a monolayer of aromatic carbon atoms covalently bound and organized in a hexagonal lattice structure (Figure 1). It has excellent mechanical strength, remarkable compatibility, a large surface area, and high electrical and thermal conductivities. Compared with graphene, graphene oxide (GO) is an oxidized graphene derivative. It contains oxygen-containing functional groups (hydroxyl, carboxyl, epoxy) across its lattice (Figure 1). These functional groups confer GO with unique properties such as water dispersibility, chemical tunability, and strong adsorption capacity for polar polymers or molecules. Due to these properties, graphene oxides are widely utilized in biomedical fields, including targeted drug delivery, bioimaging, biosensors, and tissue engineering. Beyond biomedical fields, GO also finds applications in electronics, such as flexible screens and solar cells, in energy storage, including batteries and supercapacitors, and in environmental applications, including water treatment.
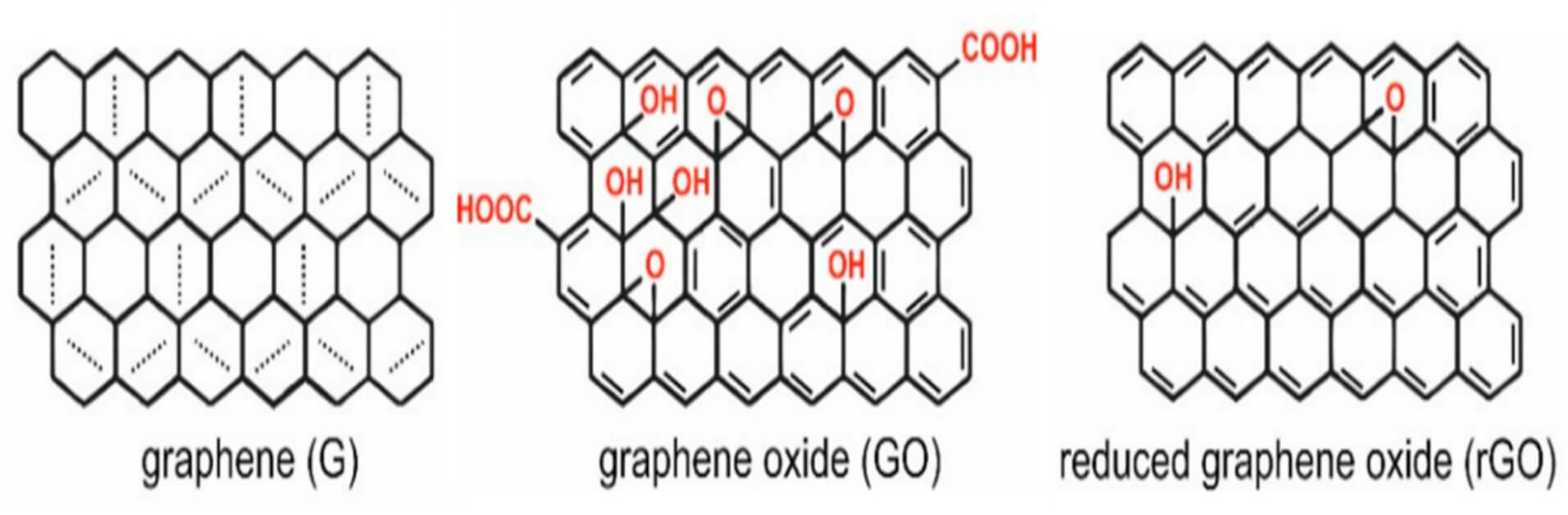 Fig.
1: The graphene (G) and graphene oxide (GO) structures1.
Fig.
1: The graphene (G) and graphene oxide (GO) structures1.
Market Size and Industry Growth Trends
The global graphene oxide market is currently experiencing significant growth. The global graphene oxide market is valued at $243.1 million in 2024, and is projected to reach $327.3 million in 2025 and $2.75 billion by 2032, with a compound annual growth rate (CAGR) of 35.5%. The fast-growing market is likely driven by advancements in nanotechnology and the expanding applications of GO in biomedical fields and various industries, including electronics and energy sectors.
Graphene Oxide Properties
As the introduction mentioned, GO is a two-dimensional carbon lattice with defects and functional oxygen groups. Carboxylic groups are usually present on the edges of GO, whereas epoxide and hydroxyl groups are located on the basal plane of GO (Figure 1). These structural features bestow GOs with various biochemical properties, making GOs particularly attractive for multiple biomedical and industrial applications.
Key properties of GOs:
- Structural basis: In GO, the sp²-bonded hexagonal carbon lattice forms a continuous two-dimensional network with high in-plane stiffness and tensile strength.
-
Functions:
- Allows GO-based films to act as robust carriers or coatings that resist tearing, swelling, or delamination in physiological environments;
- Reinforces hydrogels or tissue scaffolds;
- Improves the mechanical stability of stents or implant coatings.
- Structural basis: the extended GO sheet offers a large surface area for interactions.
-
Functions:
- Enables high drug-loading capacity.
- Structural basis: The carbon sheet of GO is decorated with abundant oxygenated groups (carboxyl, hydroxyl, epoxy), making it readily dissolve or disperse in water or physiological buffers.
-
Functions:
- Allows the production of stable suspensions that are compatible with aqueous layer-by-layer (LbL) systems.
- Structural basis: The reactive oxygen groups (carboxyl, hydroxyl, epoxide) of GOs allow covalent or noncovalent attachment of targeting ligands, such as polymers and peptides.
-
Functions:
- Permits tailored drug carriers;
- Improves biocompatibility and circulation time.
- Structural basis: GO is naturally negatively charged and can be positively charged by amine functionalization.
-
Functions:
- Allows fine-tuning of the layer composition and drug-release kinetics by alternating charged layers.
- Structural basis: The diffusion is slowed or blocked as the GO sheets are stacked densely.
-
Functions:
- Allows GOs to act as capping layers to delay or sequence drug release over weeks, hence enabling time-controlled and multi-drug delivery.
- Structural basis: Graphitic domains have high near-infrared (NIR) absorption. After absorption, they can convert NIR light to heat.
-
Functions:
- Allows for non-invasive, localized heating for photothermal cancer therapy, the dissolution of Aβ plaques in Alzheimer's disease, and skin phototherapy.
- Structural basis: Incorporating Fe₃O₄ or other iron oxide nanoparticles onto GO sheets imparts responsiveness to external magnetic fields.
-
Functions:
- Facilitates magnetically guided drug delivery;
- Enhances MRI contrast;
- Supports combined magnetic–photothermal therapies.
- Structural basis: The toxicity of GOs depends on size, dose, and functional coatings.
-
Functions:
- Allows safe systemic delivery and implant coatings (e.g., stents) by improved corrosion resistance, reduced inflammatory response, and enhanced cell adhesion via functional coatings.
Graphene Oxide Synthesis
Graphene oxide can be synthesized using four basic methods: the Staudenmaier, Hofmann, Brodie, and Hummers methods. The most common method of GO production is the Hummers method, which oxidizes graphite using concentrated acids and strong oxidants. Although this method is safe and efficient, it generates toxic gases, including nitrogen dioxide, dinitrogen tetroxide, and chlorine dioxide. Recent method development focuses on eco-friendly synthesis. For instance, a modified Hummer's method is being developed to reduce environmental impact, increase scalability, and enhance biomedical safety. The modified Hummers method is described below (Figure 2):
-
Initial Mixture Preparation
Graphite is mixed with concentrated H₂SO₄ and NaNO₃ in a ratio of about 1:25:0.5. In this step, the acid intercalates and protonates the graphite layers. At the same time, nitrate ions expand and partially oxidize them, creating space for later oxidation. -
Controlled KMnO₄ Addition
KMnO₄ is carefully added to the treated graphite (about 2–5 parts per 1 part graphite) while keeping the mixture near 20 °C in an ice bath. In this step, KMnO₄ acts as the primary oxidizing agent, introducing oxygen functionalities into the graphite layers. At the same time, the low temperature prevents excessive heat and preserves the carbon lattice for controlled oxidation. -
Quench & Heat
Distilled water is carefully added at a ratio of approximately 50 parts to 1 part graphite, while maintaining a temperature below 20 °C. Then, 150 more parts are added, and the temperature is heated to 95 °C. In this step, the first water addition safely quenches the excess oxidant, and the subsequent dilution with heating drives the oxidation to completion, exfoliating the graphite into a dark brown graphene oxide slurry. -
Final Oxidation & Purification
Approximately 10 parts of 30% H₂O₂ are added to 1 part graphite, and the mixture is stirred until the suspension turns bright yellow. Then the suspension is repeated washed with 5% HCl and distilled water until the solution is neutral. In this step, hydrogen peroxide removes manganese residuals, and the acid washes remove metal salts, leaving purified graphene oxide. -
Drying
The product is dried at 60 °C for 24 h to obtain a stable and storable GO powder.
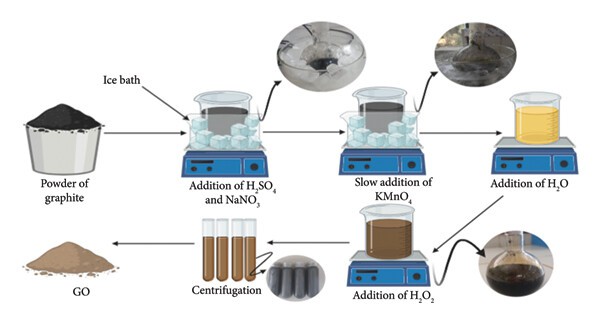 Fig.
2 Step-by-step of graphene oxide (GO) synthesis by modified Hummers4.
Fig.
2 Step-by-step of graphene oxide (GO) synthesis by modified Hummers4.
GO Functionalization for Drug Delivery
To optimize GO as a drug delivery nanocarrier, functionalization of GOs is applied to increase solubility, biocompatibility, drug-loading, and release efficiency, and cell-targeting ability (Figure 3). GO functionalization can be generally divided into covalent, noncovalent, and hybrid (combined) approaches, each with distinct mechanisms and applications.
Covalent Functionalization: Stable and Permanent Modification
Covalent bonding refers to the covalent attaching of molecules (i.e., polymers, peptides, or drugs) to the oxygen-containing groups (e.g., carboxyl or hydroxyl) on the GO surface through chemical reactions. It forms strong and stable modifications, making it suitable for applications requiring durability (e.g., long-term drug delivery).
Key Mechanisms & Examples:
- Amide Bond Formation: Crosslinkers bind amine-containing molecules (e.g., PEG-NH₂, chitosan) to GO carboxyl groups. In the cytotoxicity assay performed on fibroblast cells, PEGylated GO (NGO-PEG) is seen to decrease in vitro cytotoxicity by 40–60% relative to raw GO.
- Silane Modification: Silane agents (e.g., APTES) react with GO's hydroxyl groups to introduce amino or epoxy groups, enabling further conjugation with drugs or targeting ligands.
- Polymer Grafting: Covalently attaching biocompatible polymers (e.g., polyacrylic acid/PAA, and polyacrylamide/PAM) to the surface of GO enhances its mechanical strength and drug-loading efficiency. For instance, NGO-PAA shows 2x higher loading capacity of hydrophilic drugs than unmodified GO.
Advantages:
- Stable conjugation (resists dissociation in biological fluids).
- Precise control over surface chemistry (e.g., targeting ligand density).
Noncovalent Functionalization: Gentle and Flexible Modification
Noncovalent functionalization employs weak intermolecular interactions (π-π stacking, hydrophobic interactions, hydrogen bonding) to attach molecules to the basal plane of GO. This strategy maintains the pristine properties of GO (e.g., electrical conductivity) and is well-suited for sensitive cargos (e.g., natural therapeutics, proteins).
Key Mechanisms & Examples:
- π-π Stacking: GO's hexagonal carbon lattice can bind to aromatic molecules (e.g., curcumin, doxorubicin, plant-derived phenolics). In the tumor treatment, curcumin-loaded GO via π-π stacking can achieve a drug content of 4.5–29 wt% and exhibit pH-responsive release.
- Hydrophobic Interactions: Hydrophobic segments of molecules (e.g., lipids, essential oils) can be attached to GO's nonpolar basal plane, thereby improving the solubility of water-insoluble drugs. For example, compared with the 35% release efficiency of free drugs (a hydrophobic flavonoid), the release efficiency was increased to 91% when the hydrophobic flavonoid was loaded onto PVP-functionalized GO via hydrophobic forces.
- Hydrogen Bonding: GO's hydroxyl or epoxy groups can form hydrogen bonds with molecules such as dextran or peptides, thereby enhancing biocompatibility.
Advantages:
- No harsh chemicals (preserves natural therapeutic potency).
- Easy to scale (no complex synthesis steps).
Hybrid Functionalization: Synergizing Covalent & Noncovalent Bonds
Hybrid approaches combine both strategies to maximize GO's performance.
Examples:
- Dual-Drug Delivery: PEGylated GO (covalent amide bonds with PEG-NH2) loads cisplatin (covalent) and doxorubicin (noncovalent π-π stacking). This system achieves 36.7–37.6% drug loading and 64.6–65.7% controlled release, with synergistic anticancer effects in MCF-7 cells.
- Targeted Antimicrobial Systems: Aptamer-conjugated GO (covalent bonding) loads berberine (noncovalent π-π stacking) to target MRSA. The hybrid system reduces bacterial viability by 99.9% and prevents the formation of biofilms.
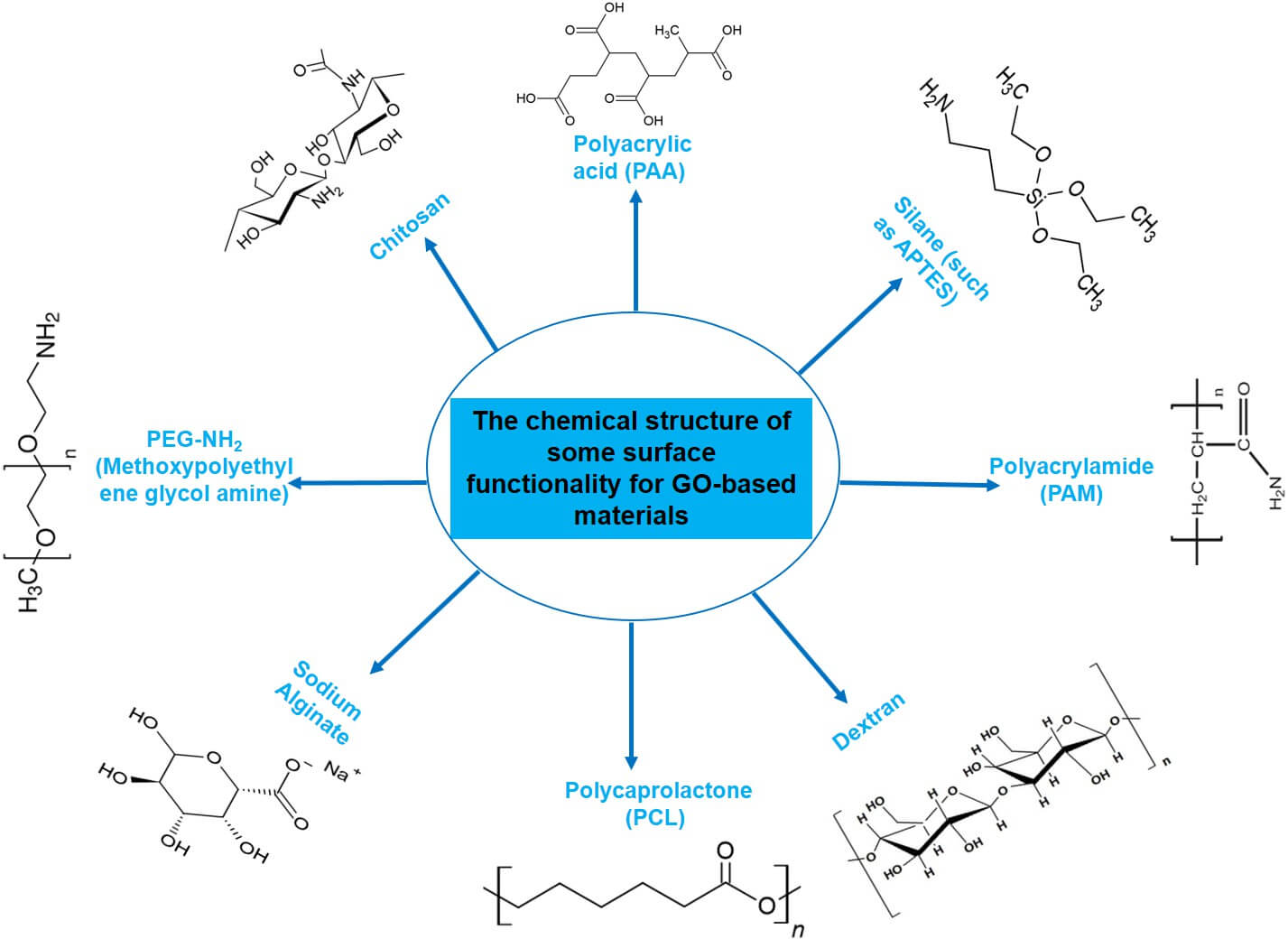 Fig.
3 The functionalization of GO6.
Fig.
3 The functionalization of GO6.
Graphene Oxides in Biomedicine
Graphene oxides (GOs) are endowed with multiple properties that have led to the development of a wide range of biomedical applications. The following represent some of the major areas where GOs have found their application.
Drug and gene delivery
Graphene Oxide (GO) has a large surface area, colloidal stability, and tunable loading/ release property; thus, it can serve as a potential nanocarrier for drug delivery. Therapeutic drugs can be loaded onto GOs via π-π stacking or hydrophobic interactions with pH- or photo-responsive release. In gene delivery, functionalized GOs have been used to deliver siRNA/plasmid DNA to target tumor cells, thus inhibiting their growth.
Phototherapy
Ligand-mediated targeting utilizes specific interactions between receptors and ligands to direct LNPs to the target cells/tissues. Two primary methods are applied for attaching ligands to LNPs: post-insertion and direct conjugation.
Bioimaging
GOs are fluorescent-emitting upon excitation at specific wavelengths (350–650 nm) for optical imaging. GOs can also serve as carriers of contrast agents (e.g., gadolinium-decorated graphene oxides) for magnetic resonance imaging (MRI) or photoacoustic imaging.
Biosensing
GOs can be used for detection either through fluorescence quenching or by their electrical properties. Interactions with single-stranded DNA (ssDNA) allow for sequence-specific recognition. The surface of GO can be functionalized with various biomolecules that have high affinity for a specific biomarker, enabling detection and, thus, diagnostic use.
Tissue engineering
GOs/rGOs can help with tissue regeneration via supporting stem cell proliferation and differentiation. The incorporation of GOs/rGOs in scaffolds can improve their electroconductivity, which is essential for cardiac and nerve regeneration. GO-incorporated poly (lactic-co-glycolic acid) (PLGA) scaffolds, for example, have been shown to support better mesenchymal stem cell growth.
Antibacterial applications
GOs could damage the bacterial membrane, which subsequently results in oxidative stress, thus suppressing the growth of pathogens (such as E. coli and S. aureus).
GO-Based Drug Delivery Strategies
Passive Targeting
Because tumor blood vessels are leaky (with pores of 400–800 nm), GO nanoparticles can exploit the enhanced permeability and retention (EPR) effect, thereby allowing for GO accumulation in tumor tissues. In this way, the therapeutic efficacy of encapsulated drugs is improved significantly.
Active Targeting
By conjugating GO with specific ligands, such as antibodies or peptides, it becomes possible to direct the nanoparticles to particular cell types or tissues, improving specificity and minimizing off-target effects.
Controlled Drug Release Systems
GO can be engineered to release its payload in response to specific stimuli, such as changes in pH, temperature, or the presence of certain enzymes. This allows for controlled and sustained release of the drug.
Co-delivery of Multiple Therapeutics
GO's large surface area allows for the simultaneous loading of multiple therapeutic agents, allowing for combination therapies, such as therapies that target different pathways.
Other Applications of Graphene Oxides
GO's versatility enables applications across the industrial field.
Catalysis
GO has been used as a catalyst or catalyst support in a wide range of organic reactions and cross-couplings. The presence of oxygen groups can provide active sites for the binding and activation of reactants. In composites, GO is used to improve the catalytic activity of other materials; for example, the use of a GO–metformin–Cu composite achieved yields of 99% in a model reaction.
Photocatalysis
GO can be used for the degradation of pollutants, hydrogen production from water splitting, and carbon dioxide reduction. Its band gap is also tunable to absorb a broad range of light for photocatalytic applications.
Electrochemistry
GO has been used in electrochemical applications such as hydrogen evolution and biosensing. The material's conductivity and high surface area can promote efficient electron transfer in electrochemical reactions.
Environmental Remediation
GO can be used in environmental remediation due to its high surface area and pore structure, which can adsorb heavy metals and degrade volatile organic compounds (VOCs).
Optoelectronics
In the optoelectronics field, GO has been used to improve the electrodes of solar cells. The material can also be used for the sensitive detection of pollutants through fluorescence quenching and Raman enhancement.
Future Directions in Graphene Oxide Research
Efficacy and Safety Evaluation
Although many studies on GO nanocarriers show promising therapeutic effects, a systematic evaluation of their long-term toxicity, biodistribution, and elimination pathways is required. Only with sufficient preclinical and clinical evidence can GO-based nanocarriers move toward regulatory approval and patient use.
Sustainable Production
The production of GO using traditional methods consumes large amounts of strong oxidants and acids, resulting in hazardous waste. In the future, green chemistry will be prioritized, and a scalable and eco-friendly production of GO will be developed by adopting milder oxidants, recyclable solvents, and low-energy processes.
Hybrid Nanomaterials
Multifunctional nanoplatforms that are capable of performing simultaneous drug delivery, imaging, sensing, and even catalysis can be created by combining GO with metals, polymers, or biomolecules. These hybrids will enable simultaneous drug delivery and magnetic or optical tracking of the nanoparticles within the body.
Flexible Electronics
GO can be integrated with wearable biosensors and other flexible electronics. These integrated devices can allow for continuous monitoring of health indicators, such as glucose levels and cardiovascular signals.
Personalized Medicine
Based on patient-specific information, it would be possible to tailor GO-based nanocarriers to optimize drug delivery parameters, including dose, timing, and targeting, thereby achieving proximal efficacy with reduced side effects.
FAQs
What is graphene oxide?
Graphene oxide is an oxidized form of graphene, containing oxygen functional groups that make it water-dispersible and chemically versatile.
What are the side effects of graphene oxide in humans?
Though low concentrations are generally well-tolerated, GO may cause oxidative stress and inflammation at high doses.
What is the difference between graphene and graphene oxide?
As Table 1 illustrates, graphene and graphene oxide differ in terms of their structure, conductivity, solubility, surface functionalization, and applications.
Table (Oliveira et al.) Comparison between graphene and graphene oxide.
| Feature | Graphene | Graphene Oxide |
|---|---|---|
| Structure | Pure carbon sheet | Oxygen-functionalized carbon sheet |
| Conductivity | High | Lower, tunable |
| Solubility | Hydrophobic | Hydrophilic |
| Surface Functionalization | Limited | Extensive |
| Applications | Electronics, composites | Drug delivery, biosensors |
What is reduced graphene oxide, and how is it used in delivery?
Reduced graphene oxide (rGO) is obtained by chemically reducing GO, thus partially restoring some of its electrical conductivity. rGO can be utilized in drug delivery systems that require electrical responsiveness or enhanced conductivity.
Our Commitment
At Creative Biolabs, we provide customized services that enable researchers and industry innovators to advance their GO-based biomedical solutions.
Related Products and Services You May Be Interested in
Graphene Oxide based Delivery System
References
- Oliveira, A. M. L. et al. "Graphene Oxide Thin Films with Drug Delivery Function". Nanomaterials 12, 1149 (2022). https://www.mdpi.com/2079-4991/12/7/1149. Distributed under Open Access license CC BY 4.0 , without modification.
- Žárská, L. et al. "Dual Drug Delivery in Cancer Therapy Using Graphene Oxide‐Based Nanoplatforms". Advanced NanoBiomed Research 2400026 (2024) doi:10.1002/anbr.202400026. https://onlinelibrary.wiley.com/doi/10.1002/anbr.202400026.
- Yaghoubi, F. et al. "A functionalized graphene oxide with improved cytocompatibility for stimuli-responsive co-delivery of curcumin and doxorubicin in cancer treatment". Sci Rep 12, 1959 (2022).https://www.nature.com/articles/s41598-022-05793-9.
- Bourachdi, S. E. et al. "Enhancing Graphene Oxide Production and Its Efficacy in Adsorbing Crystal Violet: An In‐Depth Study of Thermodynamics, Kinetics, and DFT Analysis". International Journal of Chemical Engineering 2024, 8222314 (2024). https://onlinelibrary.wiley.com/doi/10.1155/2024/8222314. Distributed under Open Access license CC BY 4.0 , without modification.
- Bellier, N., Baipaywad, P., Ryu, N., Lee, J. Y. & Park, H. "Recent biomedical advancements in graphene oxide- and reduced graphene oxide-based nanocomposite nanocarriers". Biomater Res 26, 65 (2022). https://spj.science.org/doi/10.1186/s40824-022-00313-2.
- AbouAitah, K., Sabbagh, F. & Kim, B. S. "Graphene Oxide Nanostructures as Nanoplatforms for Delivering Natural Therapeutic Agents: Applications in Cancer Treatment, Bacterial Infections, and Bone Regeneration Medicine". Nanomaterials 13, 2666 (2023). https://www.mdpi.com/2079-4991/13/19/2666. Distributed under Open Access license CC BY 4.0 , without modification.
Created in September 2025



