Gold Nanoparticle-Based Delivery Strategies: From Mechanism to Commercial Availability
Gold nanoparticle-based delivery strategies are rapidly reshaping how modern drugs, genes, and imaging agents are designed and targeted. Because these ultra-small particles combine tunable size, strong optical properties, and easy surface modification, they enable more precise delivery than many traditional systems. In this article, Creative Biolabs will walk you through the mechanisms, applications, and emerging commercial landscape behind these powerful delivery technologies.
What Are Gold Nanoparticles?
Gold nanoparticles (often called AuNPs) are tiny particles of metallic gold that usually range from 1 to 100 nanometers in size. At this scale, gold behaves very differently from bulk metal. Because of their unique properties, gold nanoparticle-based delivery strategies are now a key part of nanomedicine and targeted drug delivery.
Several simple but powerful features make gold nanoparticles attractive for drug delivery:
- High surface-to-volume ratio – they can carry many drug molecules, ligands, or polymers on a single particle.
- Surface plasmon resonance – they interact strongly with light, which enables imaging and photothermal therapy.
- Easy surface functionalization – chemistries like thiol-gold binding make it simple to attach drugs, antibodies, peptides, or nucleic acids.
- Tunable size and shape – by changing synthesis conditions, you can make spheres, rods, shells, cages, or stars.
Because of this flexibility, gold nanoparticle-based delivery strategies can be tailored for different drugs, routes, and disease targets, especially in oncology.
How Gold Nanoparticles Work in Drug Delivery
Gold nanoparticle-based delivery strategies rely on how the particles move, where they accumulate, and how they release the payload. Several mechanisms usually work together (Figure 1).
1. Passive Targeting (EPR Effect)
Tumors often have leaky blood vessels and poor lymphatic drainage. This is known as the enhanced permeability and retention (EPR) effect.
- Gold nanoparticles sized around 10-100 nm can:
- Leak out of tumor blood vessels more easily than small molecules.
- Stay in the tumor space longer than in healthy tissue.
Therefore, even without specific ligands, gold nanoparticle-based delivery strategies can passively enrich drugs in tumors compared with free drugs.
2. Active Targeting Using Ligands
To further improve selectivity, scientists use active targeting. In this approach, the surface of the gold nanoparticle is decorated with recognition ligands, for example:
- Monoclonal antibodies (e.g., against HER2 or EGFR).
- Peptides (such as RGD motifs that recognize integrins).
- Folate or other small molecules that bind to overexpressed receptors.
- Aptamers that bind specific proteins or cells.
With these ligands, gold nanoparticle-based delivery strategies can:
- Bind more strongly to tumor cells or disease-related cells.
- Increase local uptake and reduce off-target exposure.
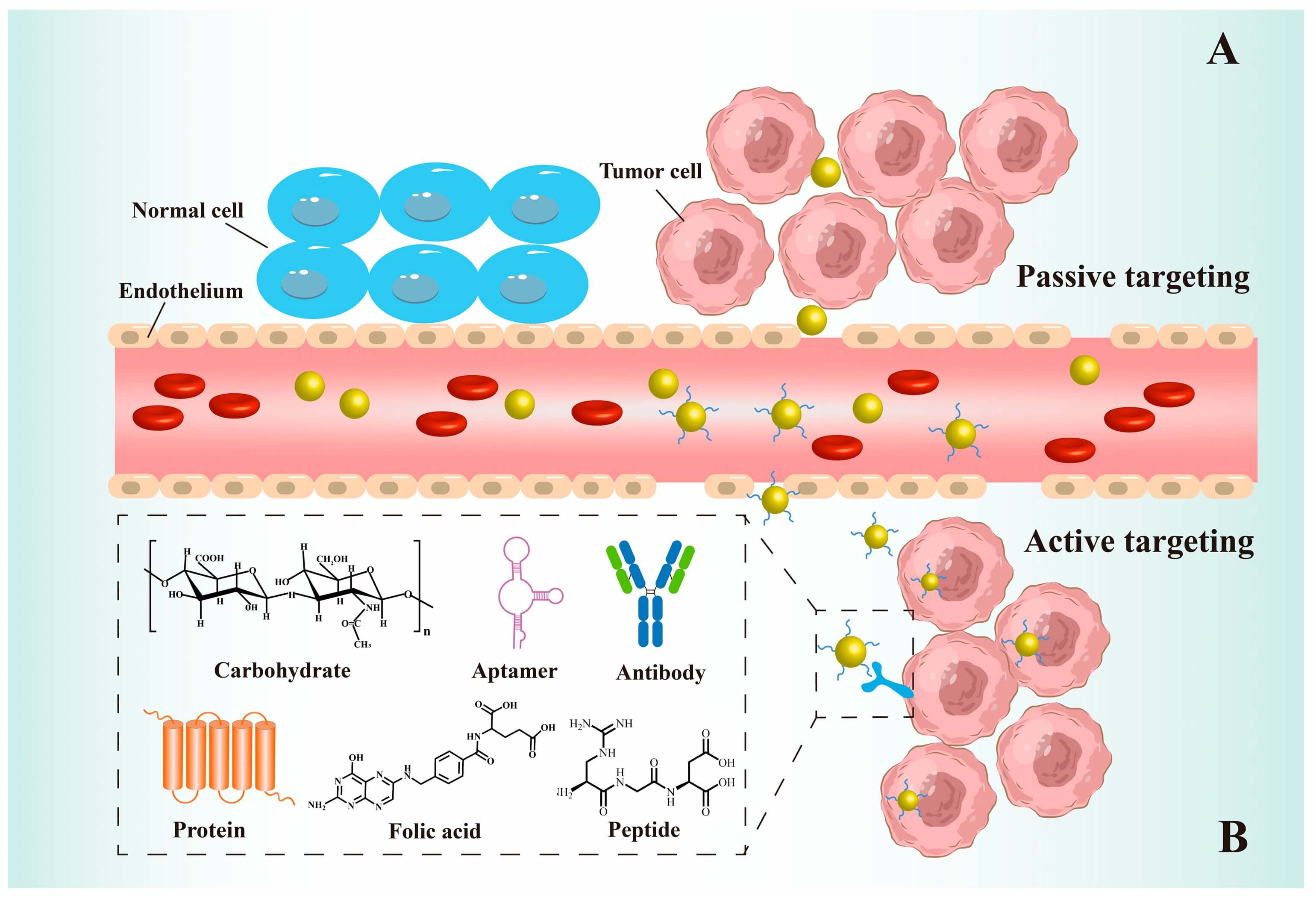 Fig.1
The targeting mechanisms of AuNP-based delivery systems.1
Fig.1
The targeting mechanisms of AuNP-based delivery systems.1
3. Stimuli-Responsive Release
To avoid premature release, many systems are designed to respond to specific triggers(Figure 2):
- pH-sensitive linkers that break in acidic tumor or endosomal environments.
- Redox-responsive bonds (e.g., disulfides) that cleave in the high-glutathione intracellular space.
- Near-infrared (NIR) light that heats the gold nanoparticle and triggers drug release.
- Enzyme-sensitive coatings that degrade in disease microenvironments.
Because gold can efficiently convert NIR light into heat, photothermal effects are often combined with controlled release in a single gold nanoparticle-based delivery strategy.
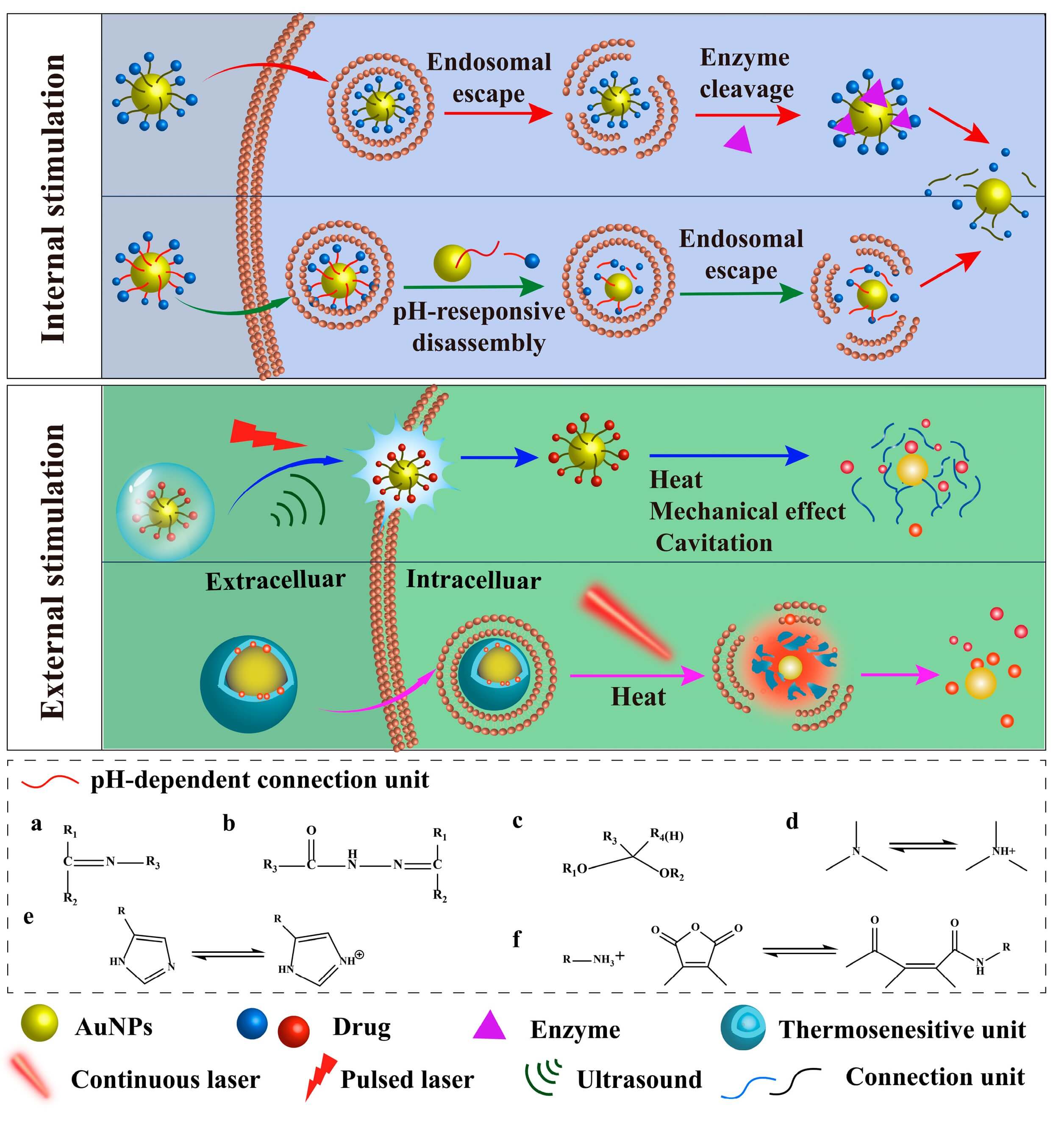 Fig.2
The stimuli-responsive AuNP-based delivery systems.1
Fig.2
The stimuli-responsive AuNP-based delivery systems.1
4. Conjugation Chemistries
How a drug is attached to the gold nanoparticle is just as important as the drug itself. Common approaches include:
- Thiol-gold bonds for stable attachment of thiolated ligands, peptides, or oligonucleotides.
- Polymer coatings like PEG, chitosan, or other biocompatible polymers that can carry drugs in or on the coating.
- Electrostatic adsorption for charged small molecules or nucleic acids.
By tuning these chemistries, you can control:
- Stability in blood
- Release rate
- Cellular uptake behavior
For additional options that integrate with these mechanisms, explore our advanced Targeted Delivery Module Systems.
Gold Nanoparticles in Cancer Therapy
Cancer remains the main application area for gold nanoparticle-based delivery strategies. Patients and researchers often search for "gold nanoparticles in cancer treatment" because these platforms bring imaging, targeting, and therapy together.
Photothermal Therapy (PTT)
In photothermal therapy, gold nanoparticles absorb NIR light and convert it into heat. When they are located in a tumor, this heat can:
- Damage or kill tumor cells locally.
- Spare surrounding healthy tissue when light is well focused.
Shapes like gold nanorods, nanoshells, and nanocages are engineered for strong NIR absorption, which supports deeper tissue penetration and more efficient tumor ablation.
Chemotherapy + AuNP Conjugates
Gold nanoparticles can also carry classic chemotherapy drugs, such as doxorubicin or paclitaxel:
- The drug can be attached via cleavable linkers.
- It can be co-delivered with targeting antibodies.
- It can be combined with photothermal therapy for a "double hit".
Several preclinical studies show that antibody-drug-AuNP conjugates can:
- Increase tumor selectivity.
- Enhance cytotoxicity in target cells.
- Reduce systemic toxicity compared with the free drug.
Theranostics (Imaging + Therapy)
Because gold offers strong X-ray and optical contrast, gold nanoparticle-based delivery strategies lend themselves to theranostics, where:
- The same nanoparticle is used for imaging (e.g., CT or photoacoustic imaging).
- And for therapy (e.g., drug delivery or photothermal ablation).
This combined approach helps researchers:
- Track where the nanoparticles go.
- Adjust dosing and timing in real time.
- Improve precision in image-guided interventions.
Hybrid and Multi-Modal Gold Nanoparticle Systems
Gold nanoparticles can also be combined with other nanocarriers to create hybrid systems.
AuNP–Liposomes
Here, gold nanoparticles are:
- Embedded in or attached to liposomal membranes.
- Used to add imaging or photothermal functions to classic liposomal drug delivery.
AuNP–Polymer Nanoparticles
AuNPs can be:
- Co-loaded with drugs inside a polymer matrix.
- Used to trigger thermal or redox-based release from polymer shells.
AuNP–Hydrogel and Implantable Platforms
Hydrogels and local implants can:
- Hold gold nanoparticles at a specific site, such as a tumor bed or surgical cavity.
- Release both heat and drugs in a localized, controlled way.
Combining Chemo + PTT + Imaging in One Construct
In many designs, gold nanoparticle-based delivery strategies are fully multi-modal, offering:
- Chemotherapy payloads for cytotoxic effects.
- Photothermal therapy for local ablation.
- Imaging contrast for treatment planning and monitoring.
For readers interested in broader nanocarrier options, you can explore the Targeted Delivery Module Systems offered by Creative Biolabs, which integrate different delivery platforms beyond gold nanoparticles.
Clinical and Translational Status
Several gold nanoparticle formulations have entered early-phase clinical trials for:
- Solid tumor imaging.
- Radiosensitization.
- Photothermal therapy.
However, most candidates are still in the translational or clinical-development stage, not in broad clinical use. Challenges include:
- Demonstrating clear benefit over existing nanocarriers like liposomes or polymeric nanoparticles.
- Addressing concerns about long-term retention and organ accumulation.
- Meeting strict regulatory expectations for manufacturing and characterization.
Even so, as nanomedicine advances, gold nanoparticle-based delivery strategies remain a promising part of the pipeline, especially in oncology and precision medicine.
Design Considerations for AuNP Therapeutics
The success of a gold nanoparticle-based delivery strategy depends heavily on smart design choices.
1. Size (10-100 nm)
- Smaller particles can circulate longer and may cross some biological barriers more easily.
- Larger particles may show stronger EPR-based tumor accumulation but different clearance paths.
Typical design targets lie between 10 and 100 nm, balancing uptake, circulation, and clearance.
2. Shape (Spheres, Rods, Stars, Cages)
Different shapes provide different optical and biological behaviors:
- Spheres – simple, well-characterized, widely used.
- Rods – strong NIR absorption, ideal for photothermal therapy.
- Stars and cages – high surface area and tunable plasmonic properties.
3. Surface Chemistry
Surface chemistry controls:
- Stability in blood
- Recognition by the immune system
- Non-specific protein adsorption
Common strategies include:
- PEGylation to reduce opsonization and extend circulation.
- Biomimetic coatings (e.g., cell membranes, serum proteins) to improve stealth and targeting.
4. Targeting Ligands (RGD, Antibodies, Aptamers, Peptides)
Finally, ligands add specificity. For example:
- RGD peptides for integrin-rich tumors.
- Antibodies against HER2, EGFR, or other overexpressed receptors.
- Aptamers and peptides matched to unique tumor markers.
Safety, Toxicity, and Regulatory Considerations
Although elemental gold is often seen as inert and biocompatible, safety is not automatic for all gold nanoparticle-based delivery strategies. Key factors include:
- Size and shape – very small particles may cross more barriers and accumulate.
- Surface charge – highly cationic surfaces may damage cells.
- Coating materials – some polymers or ligands may be immunogenic.
- Dose and schedule – repeated dosing can lead to a buildup in the liver, spleen, or kidneys.
Regulators focus on:
- Biodistribution and clearance data.
- Long-term retention in organs and potential toxicity.
- Batch-to-batch consistency under GMP conditions.
Because of these requirements, it is essential to pair innovation with robust safety testing and a clear regulatory strategy.
To support these design and safety considerations, you can also explore our specialized Targeted Delivery Module Systems for customizable nanoparticle engineering.
Manufacturing Challenges for Medical-Grade AuNPs
Manufacturing medical-grade gold nanoparticles requires far more precision than producing standard research materials, which is why scaling them for clinical use presents unique technical and economic challenges.
Why Medical Gold Nanoparticles Are Expensive
Medical-grade gold nanoparticles cost more than bulk gold because of:
- High-purity starting materials.
- Narrow size distribution control.
- Strict cleaning and quality-control requirements.
However, each nanoparticle can carry a very high amount of drug per unit of gold, so the actual gold cost is often a small fraction of the final therapy cost.
GMP Considerations
Manufacturing for clinical use must follow GMP standards, which cover:
- Controlled synthesis and purification.
- Detailed characterization (size, charge, shape, purity).
- Documentation and traceability.
Industrial Supply Chain and Leading Vendors
Today, several chemical and life-science suppliers offer:
- Research-grade gold nanoparticles.
- Custom synthesis services.
Partnerships between such vendors, pharma companies, and CROs help move gold nanoparticle-based delivery strategies toward the clinic.
Scalability Gaps in Photothermal & Targeted Therapies
Still, scalability remains challenging, especially for:
- Complex multi-ligand, multi-payload nanoparticles.
- Highly shape-specific constructs like rods or cages.
Close collaboration with experienced development partners is essential to bridge the gap between lab-scale proof-of-concept and scalable production.
How Creative Biolabs Supports Gold Nanoparticle Delivery Programs
Creative Biolabs provides end-to-end support for gold nanoparticle-based delivery strategies, from concept to preclinical validation. Our teams can help you:
- Design AuNPs with optimized size, shape, and composition for your indication and route.
- Engineer surface chemistry, including PEGylation, biomimetic coatings, and ligand conjugation.
- Select and attach targeting ligands, such as antibodies, peptides, or aptamers, tailored to your disease targets.
- Integrate payloads, including small molecules, peptides, proteins, and nucleic acids.
- Evaluate performance using in vitro cell-based models andin vivo animal models, including uptake, biodistribution, pharmacokinetics, and efficacy.
To explore how gold nanoparticles fit into broader targeted-delivery platforms, you can also review our Targeted Delivery Module Systems and the full Targeted Delivery Solutions portfolio.
For Research Use Only. Not for Clinical Use.
Related Services You May Be Interested in
FAQs
What are gold nanoparticles used for in drug delivery?
Gold nanoparticles are used as tiny carriers that can hold drugs, genes, or imaging agents and bring them directly to specific tissues, such as tumors. Their small size, high surface area, and easy surface modification make them ideal for targeted and controlled drug delivery.
How do gold nanoparticles target cancer cells?
They reach cancer cells through two main pathways: passive targeting using the enhanced permeability and retention (EPR) effect, and active targeting using ligands like antibodies, peptides, or aptamers. These features allow gold nanoparticle-based delivery strategies to improve drug concentration in tumors while reducing exposure to healthy tissue.
Are gold nanoparticles safe for medical use?
Gold itself is chemically inert, but the safety of gold nanoparticles depends on their size, coating, surface charge, and dose. Properly designed particles with neutral or PEGylated surfaces show better compatibility, while regulators focus on long-term clearance and organ accumulation.
What types of drugs can be delivered using gold nanoparticles?
Gold nanoparticles can carry a wide range of payloads, including small-molecule chemotherapies, peptides, proteins, siRNA, mRNA, DNA plasmids, and imaging dyes. Their flexible surface chemistry makes them compatible with many therapeutic and diagnostic molecules.
How do gold nanoparticles release their drug payload?
Release can occur through pH-sensitive linkers, redox-responsive bonds, enzyme-triggered degradation, or external triggers like near-infrared light used in photothermal therapy. These mechanisms allow controlled and localized drug release at the disease site.
Are gold nanoparticles used in clinical trials?
Yes. Several gold nanoparticle formulations have entered early clinical trials for imaging, radiosensitization, and photothermal therapy in solid tumors. While no AuNP-based therapy has full commercial approval yet, clinical interest in this technology continues to grow.
What makes gold nanoparticles useful in cancer imaging and theranostics?
Their strong optical and X-ray absorption properties allow gold nanoparticles to enhance CT imaging, photoacoustic imaging, and optical signals. When combined with drug loading or photothermal therapy, they enable theranostic systems that diagnose and treat at the same time.
Summary
Gold nanoparticle-based delivery strategies bring together passive and active targeting, precise surface engineering, and powerful photothermal and imaging capabilities, making them one of the most promising tools in modern nanomedicine. Their ability to carry diverse payloads, respond to physiological triggers, and support multi-modal cancer therapies positions them strongly in a fast-growing global market. Yet true success requires careful control of particle size, shape, coatings, ligand selection, and GMP-grade manufacturing—factors that directly influence safety, biodistribution, and therapeutic outcome.
To accelerate your gold nanoparticle program with confidence, Creative Biolabs provides end-to-end support across design, characterization, targeted delivery engineering, and in vitro/in vivo validation. Our experts can help you transform early concepts into robust, data-driven candidates ready for commercial translation. Contact Creative Biolabs today to discuss your project and unlock the full potential of next-generation gold nanoparticle delivery systems.
References
- Huang, H. et al. "Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy." Pharmaceutics 15, 1868 (2023). https://www.mdpi.com/1999-4923/15/7/1868. Distributed under Open Access license CC BY 4.0, without modification.
- Mendes, R., Fernandes, A. & Baptista, P. "Gold Nanoparticle Approach to the Selective Delivery of Gene Silencing in Cancer—The Case for Combined Delivery?" Genes 8, 94 (2017). https://www.mdpi.com/2073-4425/8/3/94.
- Didamson, O. C., Chandran, R. & Abrahamse, H. A "Gold Nanoparticle Bioconjugate Delivery System for Active Targeted Photodynamic Therapy of Cancer and Cancer Stem Cells." Cancers 14, 4558 (2022). https://www.mdpi.com/2072-6694/14/19/4558.



